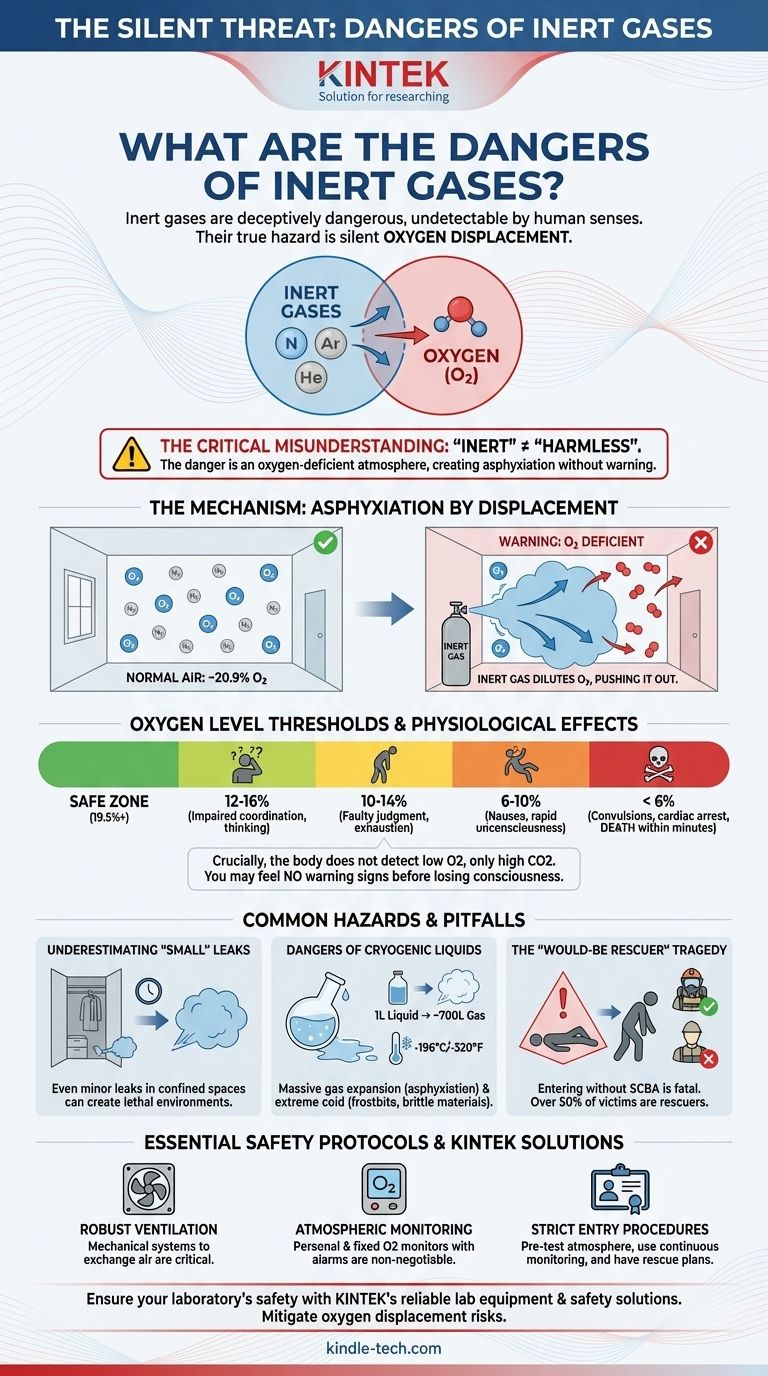
Inert gases are deceptively dangerous because they are undetectable by human senses. Their primary hazard is not chemical reactivity or toxicity, but their ability to silently displace oxygen from the air. This process, known as simple asphyxiation, can lead to rapid loss of consciousness and death without any of the typical warning signs of suffocation, like choking or gasping for air.
The common perception of "inert" as "harmless" is a critical misunderstanding. The true danger of inert gases lies in their physical ability to create an oxygen-deficient atmosphere, a condition your body cannot detect before you lose consciousness.
The Mechanism of Inert Gas Asphyxiation
It's About Oxygen Displacement, Not Toxicity
Inert gases like nitrogen, argon, and helium do not poison the body. Instead, they dilute the concentration of breathable oxygen in an enclosed or poorly ventilated area.
Normal air contains approximately 20.9% oxygen. When an inert gas leaks into a room, it physically pushes the normal air out, lowering this percentage.
The Critical Oxygen Threshold
As the oxygen level drops, the effects on the human body become severe very quickly. OSHA defines any atmosphere with less than 19.5% oxygen as oxygen-deficient.
- 12-16% Oxygen: Breathing and pulse rate increase. Muscular coordination, attention, and thinking are impaired.
- 10-14% Oxygen: Judgment becomes faulty and emotional responses are impaired. Exhaustion occurs with any exertion.
- 6-10% Oxygen: Nausea, vomiting, and an inability to move freely occur, followed by a rapid loss of consciousness.
- Below 6% Oxygen: Convulsions, cardiac arrest, and death occur within minutes.
The Body's Deceptive Response
Crucially, the primary trigger for the human body to breathe is the buildup of carbon dioxide (CO2) in the bloodstream, not the lack of oxygen.
When you breathe in a high concentration of an inert gas, you continue to exhale CO2 normally. Because CO2 levels do not build up, your brain never receives the urgent signal that you are suffocating. You will feel no chest pain or "air hunger" and may not even realize anything is wrong before you suddenly pass out.
Common Hazards and Pitfalls
Underestimating "Small" Leaks
A very common and fatal mistake is to underestimate the impact of a small, slow leak from a cylinder or pipeline.
In a confined or poorly ventilated space—such as a small lab, storage closet, or service vehicle—even a minor leak can gradually displace enough oxygen over time to create a lethal environment.
The Dangers of Cryogenic Liquids
Many inert gases, like nitrogen and argon, are stored and transported as cryogenic liquids. These present a dual threat.
First, a small amount of cryogenic liquid expands into an enormous volume of gas. For example, one liter of liquid nitrogen expands to nearly 700 liters of nitrogen gas, rapidly creating an asphyxiation hazard.
Second, the extreme cold of these liquids (often below -196°C / -320°F) can cause severe frostbite on contact and make certain materials, like carbon steel, dangerously brittle.
The "Would-Be Rescuer" Tragedy
A recurring tragedy in inert gas incidents involves a second person dying while attempting to rescue the first victim.
Seeing a colleague collapse, a person's first instinct is to rush in to help. However, by entering the same oxygen-deficient atmosphere without a self-contained breathing apparatus (SCBA), the rescuer will quickly succumb to the same fate.
Essential Safety Protocols
Ventilation is the First Line of Defense
The primary engineering control is robust ventilation. Mechanical ventilation systems that exchange the air in a room are essential wherever inert gases are used or stored in significant quantities.
Atmospheric Monitoring is Non-Negotiable
Your senses cannot detect an oxygen-deficient atmosphere. The only reliable method is to use personal or fixed oxygen monitors. These devices will sound an alarm if the oxygen level drops to the pre-set warning threshold (typically 19.5%).
Strict Entry Procedures
Never enter a space where an inert gas leak is suspected without following strict safety protocols. This includes testing the atmosphere from outside the space before entry and using continuous monitoring while inside.
For any designated confined space, a trained attendant must be posted outside, and a formal rescue plan must be in place.
Applying This to Your Work
Your safety strategy must be tailored to your specific environment and tasks.
- If your primary focus is working in a lab: Always verify that mechanical ventilation is active and consider a fixed oxygen monitor if gas quantities are significant.
- If your primary focus is entering confined spaces: Treat every entry as a high-risk operation requiring pre-testing, continuous monitoring, and a trained attendant. Never work alone.
- If your primary focus is handling cryogenic liquids: Always wear appropriate personal protective equipment (PPE), including cryo-gloves and a face shield, and be mindful of the massive gas expansion from any spill.
- If your primary focus is incident response: Never enter a suspected oxygen-deficient area to perform a rescue without an SCBA. Your first action should be to call for trained and properly equipped emergency responders.
Understanding the silent and invisible nature of inert gases is the first and most critical step toward ensuring a safe working environment.

Summary Table:
| Oxygen Level | Physiological Effects |
|---|---|
| 12-16% | Impaired coordination, thinking, and attention. |
| 10-14% | Faulty judgment, emotional impairment, exhaustion. |
| 6-10% | Nausea, vomiting, rapid loss of consciousness. |
| < 6% | Convulsions, cardiac arrest, death within minutes. |
Ensure your laboratory's safety when handling inert gases. KINTEK specializes in providing reliable lab equipment and safety solutions. From gas handling systems to environmental monitors, we help you mitigate the risks of oxygen displacement. Protect your team and your research—contact our safety experts today to discuss your specific needs.
Visual Guide
Related Products
- Hydraulic Diaphragm Lab Filter Press for Laboratory Filtration
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
- 100L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
People Also Ask
- What are the failures in a hydraulic system? Prevent Costly Downtime with Expert Diagnosis
- What happens if a hydraulic system leaks? Prevent Costly Damage and Safety Hazards
- What are some of the problems related to hydraulic power? Manage Leaks, Contamination, and Inefficiency
- What are the disadvantages of hydraulic machines? Key Trade-offs in Power and Performance
- What is the lifespan of a filter media? Understand the 3 Types for Optimal Filtration



















