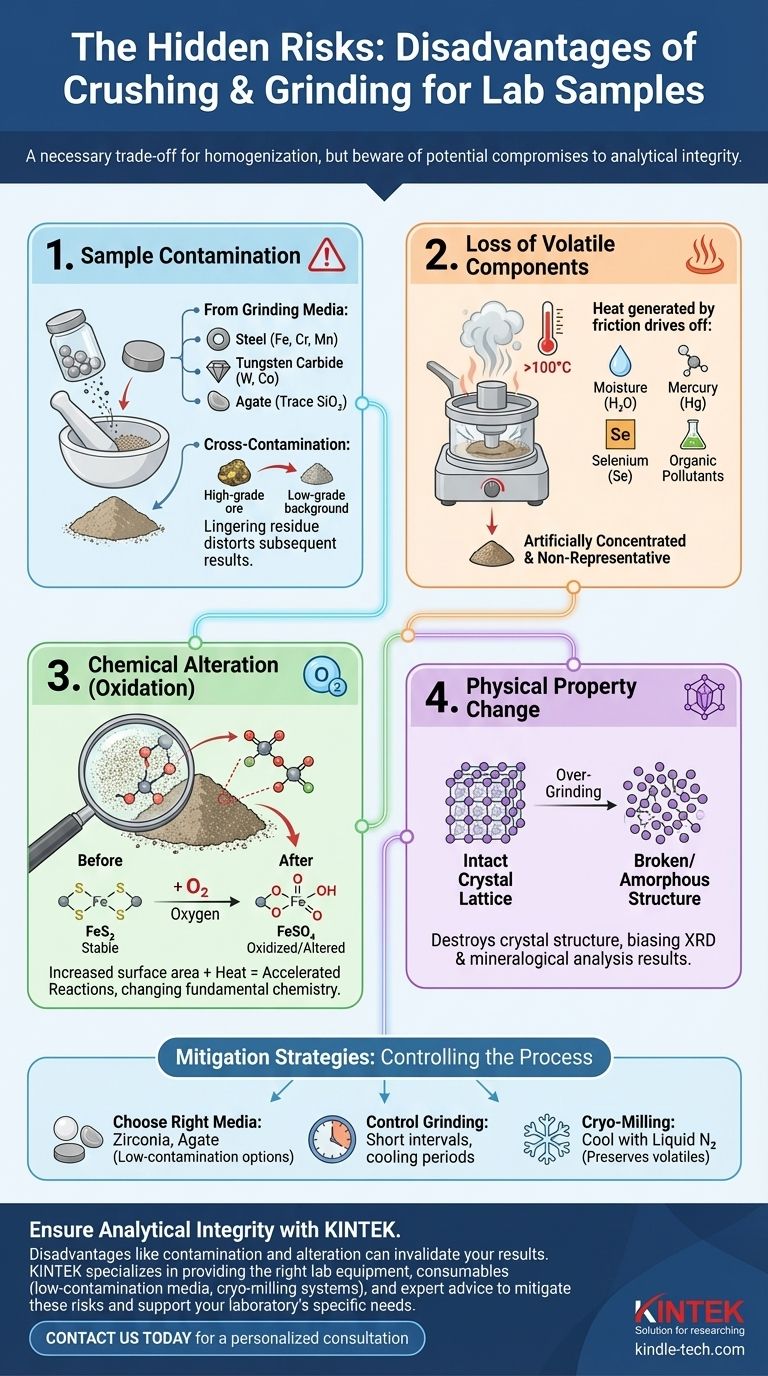
While essential for creating a uniform sample, the process of crushing and grinding a gross sample into a laboratory-ready powder is fraught with potential disadvantages. The primary drawbacks include introducing contamination from the equipment, causing the loss of volatile components due to heat, altering the sample's chemical state through oxidation, and changing its physical properties in unintended ways. Each of these can significantly compromise the integrity of the sample and the accuracy of the final analytical result.
The core challenge of sample preparation is a fundamental trade-off: mechanical size reduction is necessary to ensure a small lab sample is representative of the whole, but the very act of crushing and grinding introduces unavoidable physical and chemical changes that can distort the analytical truth you seek to measure.

The Primary Risk: Sample Contamination
Contamination is arguably the most significant and frequently encountered disadvantage of mechanical grinding. It can invalidate results, especially in trace element analysis where the contaminant concentration can exceed that of the analyte.
Contamination from Grinding Media
The grinding vessel and media (e.g., pucks, balls) are made of hard materials, but they are not infinitely durable. During the high-energy process of grinding, microscopic particles from the equipment abrade and mix with your sample.
For example, a steel mill can introduce significant amounts of iron (Fe), chromium (Cr), and manganese (Mn). A tungsten carbide mill is a common source of tungsten (W) and cobalt (Co), which is used as a binder. Even a hard agate mortar can introduce trace amounts of silica (SiO₂).
Cross-Contamination Between Samples
If equipment is not meticulously cleaned between uses, residue from a previous sample can be carried over to the next one. This is particularly dangerous when moving from a high-concentration sample to a low-concentration one.
A few lingering specks of a high-grade ore can dramatically skew the results of a subsequent background or waste rock sample, rendering the data meaningless.
Altering the Sample's Chemical State
The energy imparted during grinding is not just mechanical; a significant portion is converted into heat. This, combined with a massive increase in surface area, creates a highly reactive environment.
Loss of Volatile Components
Heat generated during vigorous grinding can easily exceed 100°C. This will drive off water (moisture content), which can artificially concentrate all other analytes.
More critically, it can cause the loss of other volatile or semi-volatile elements and compounds, such as mercury (Hg), selenium (Se), or organic pollutants. The sample you analyze is no longer representative of its original state.
Oxidation and Chemical Reactions
Grinding dramatically increases the sample's surface area, exposing fresh, reactive surfaces to the atmosphere. This, along with the heat, can accelerate oxidation.
A common example is the oxidation of sulfide minerals (like pyrite, FeS₂) into sulfate minerals (FeSO₄). This changes the sample's fundamental chemistry and can interfere with certain analytical procedures.
Understanding the Trade-offs and Mitigation
Despite these disadvantages, grinding is often a necessary step. The goal is not to eliminate it, but to control the process to minimize its negative effects.
The Necessity of Homogenization
You cannot analyze a 10-kilogram gross sample directly. Grinding it into a fine, uniform powder is the only way to ensure that a 1-gram subsample taken for analysis has the same average composition as the original bulk material. The risks of grinding are often less than the certainty of sampling error from a non-homogenized sample.
Choosing the Right Equipment
The choice of grinding media is a critical decision based on your analytical goals. You must select a material that does not contain the elements you are trying to measure at low levels.
If analyzing for trace iron, avoid steel. If your target is low-level tungsten, avoid tungsten carbide. Zirconia or agate are often chosen for being relatively low-contamination options for many, but not all, applications.
Controlling the Grinding Process
You can mitigate many disadvantages by managing the process itself. Use short grinding intervals with cooling periods in between to prevent excessive heat buildup.
For highly sensitive or volatile samples, cryogenic milling (cryo-milling), where the sample and vessel are cooled with liquid nitrogen, is an effective technique to prevent the loss of volatiles and unwanted chemical reactions.
Making the Right Choice for Your Goal
Your analytical objective dictates which disadvantages you must prioritize managing. A "one-size-fits-all" grinding protocol does not exist.
- If your primary focus is trace metal analysis: Your top priority is preventing contamination. Select grinding media carefully and implement a rigorous, documented cleaning protocol between every sample.
- If your primary focus is moisture content or volatile compounds: Your main concern is heat. Use short grinding times, consider cryo-milling, or explore methods that require less intense energy.
- If your primary focus is mineralogy or crystal structure (XRD): You must avoid over-grinding, which can destroy the crystalline structure of your minerals and bias the results.
- If your primary focus is major element composition (percent-level): The risks of minor contamination or volatile loss are less critical, but consistency is key. Use a standardized, repeatable grinding procedure for all samples to ensure comparable data.
Ultimately, a well-designed sample preparation protocol acknowledges these inherent risks and systematically controls for them, forming the foundation of any reliable analysis.
Summary Table:
| Disadvantage | Primary Risk | Common Example |
|---|---|---|
| Sample Contamination | Introduces trace elements from grinding media | Steel mill adds Fe, Cr, Mn; Tungsten carbide adds W, Co |
| Loss of Volatiles | Heat from grinding drives off moisture and compounds | Loss of Hg, Se, or organic pollutants; altered moisture content |
| Chemical Alteration | Increased surface area and heat cause oxidation | Oxidation of sulfide minerals (e.g., pyrite to sulfate) |
| Physical Property Change | Over-grinding can destroy crystal structure | Biased results for XRD or mineralogical analysis |
Ensure your lab's analytical integrity starts with proper sample preparation.
The disadvantages of crushing and grinding—like contamination and sample alteration—can invalidate your results. KINTEK specializes in providing the right lab equipment and consumables to mitigate these risks. Whether you need low-contamination grinding media (like zirconia or agate), cryogenic milling systems to preserve volatiles, or expert advice on designing a robust sample preparation protocol, we are here to support your laboratory's specific needs.
Contact us today to discuss how we can help you achieve accurate and reliable analytical results. Reach out via our contact form for a personalized consultation.
Visual Guide
Related Products
- Laboratory Single Horizontal Jar Mill
- High Energy Planetary Ball Mill Machine for Laboratory Horizontal Tank Type
- High-Energy Omnidirectional Planetary Ball Mill Milling Machine for Laboratory
- High-Energy Omnidirectional Planetary Ball Mill Machine for Laboratory
- High Energy Planetary Ball Mill Milling Machine for Laboratory
People Also Ask
- Why are alumina grinding balls preferred for Al/B4C mixing? Ensure High Purity and Efficient Homogenization
- What is the difference between a ball mill and an attritor mill? Choosing the Right Grinding Technology
- What is the difference between a bead mill and a ball mill? A Guide to Choosing the Right Grinding Technology
- What can a ball mill produce? Achieve Fine Powders and Slurries for Your Materials
- What is the particle size of XRF sample preparation? Achieve Accurate & Repeatable Results
- What is the role of laboratory stirring equipment in nZVI preparation? Achieve Stable and Uniform Nano Slurries
- What is the difference between a wet ball mill and a dry ball mill? Choose the Right Grinding Method for Your Material
- What is the role of an agate mortar and pestle in preparing sulfur and iron oxide mixtures? Ensure Purity in Research



















