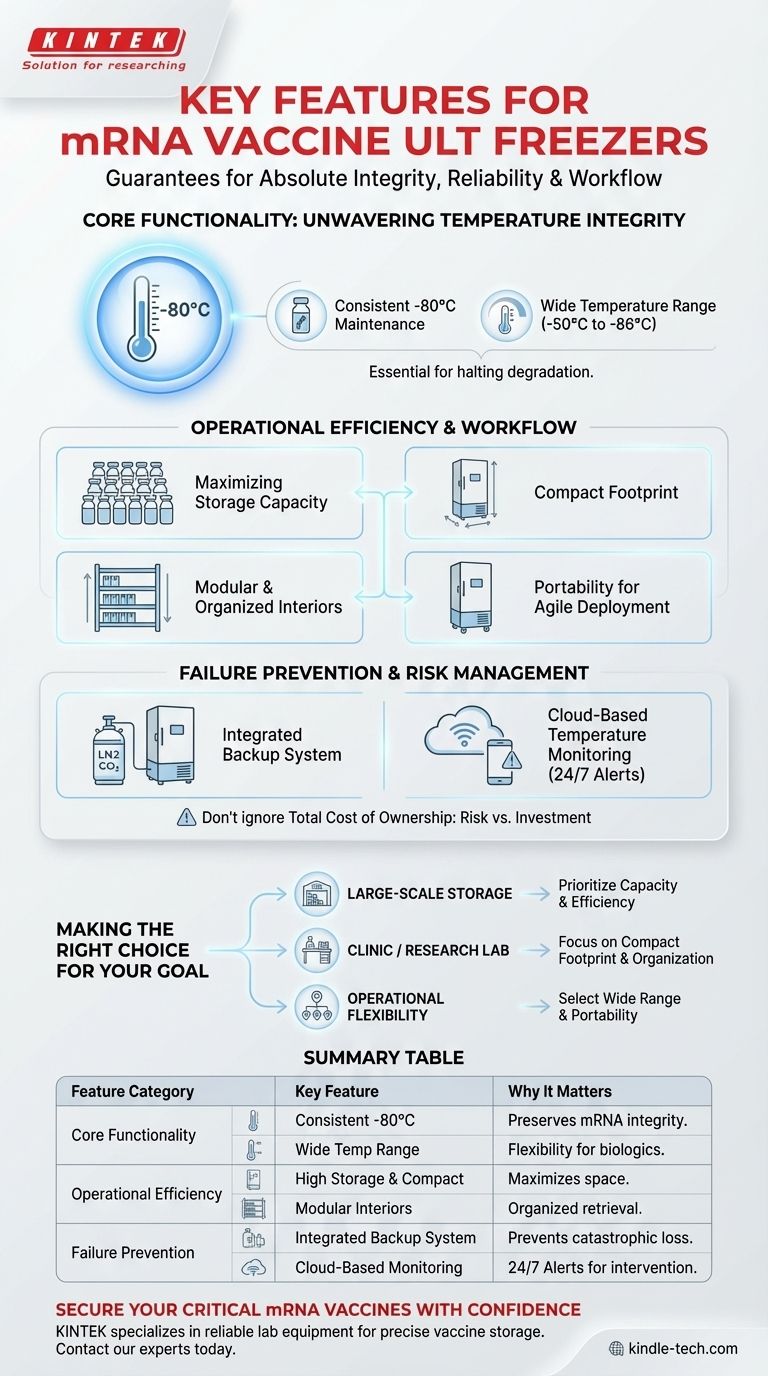
When selecting an ultra-low temperature (ULT) freezer for mRNA vaccines, you must prioritize features that guarantee absolute temperature integrity, operational reliability, and efficient workflow. Key characteristics include the ability to consistently maintain -80°C, a robust backup cooling system, integrated cloud-based temperature monitoring, and a modular interior that maximizes storage capacity within a compact footprint.
The search for the right ULT freezer is not about finding a single "best" feature. It is about building a comprehensive system of safeguards where precise temperature control, redundant systems, and real-time monitoring work together to protect priceless and irreplaceable biological assets.

Core Functionality: Unwavering Temperature Integrity
The primary function of a ULT freezer in this context is to create a stable environment that preserves the viability of mRNA vaccines. Failure is not an option.
The Critical -80°C Setpoint
mRNA molecules are notoriously fragile. The -80°C (-112°F) environment is essential to halt enzymatic processes that would otherwise degrade the vaccine, rendering it ineffective. A freezer's ability to not just reach but consistently maintain this temperature without significant fluctuation is its most critical performance metric.
Wide Temperature Range
While -80°C is the target for many mRNA vaccines, a freezer with a wider setpoint range (e.g., -50°C to -86°C) offers crucial flexibility. This allows the unit to be repurposed for other biologics, reagents, or future vaccine formulations that may have different storage requirements.
Operational Efficiency and Workflow
Beyond core temperature control, the freezer's design directly impacts day-to-day laboratory or clinical operations, safety, and inventory management.
Maximizing Storage Capacity
Freezer capacity, often measured in the number of 2-inch vials it can hold, is a key consideration for operational scale. Maximizing storage density ensures you can support your needs without consuming excessive laboratory space.
A Compact Footprint
Lab and clinical space is always at a premium. A freezer designed with a compact external footprint relative to its internal storage capacity is highly valuable, allowing for more efficient use of floor space.
Modular and Organized Interiors
A freezer with adjustable shelving and rack systems provides a modular interior. This is critical for organizing different vaccine batches, separating lots, and enabling quick retrieval. Better organization reduces the time the door is open, which in turn minimizes temperature fluctuations and protects the stored contents.
Portability for Agile Deployment
In some scenarios, such as mobile vaccination clinics or reconfiguring lab layouts, portability is a key advantage. Freezers equipped with heavy-duty casters allow for easy and safe relocation without requiring specialized equipment.
Understanding the Trade-offs and Failure Prevention
A ULT freezer is not just an appliance; it's a risk management tool. Its most important features are those that prevent catastrophic loss in the event of a component failure or power outage.
The Necessity of a Backup System
A primary compressor failure or a long-term power outage can lead to the total loss of stored vaccines. An integrated backup cooling system, often using liquid nitrogen (LN2) or carbon dioxide (CO2), is a non-negotiable feature. It automatically engages to keep the contents at a safe temperature until the primary issue is resolved.
Cloud-Based Temperature Monitoring
On-site checks are insufficient for protecting high-value assets. Cloud-based monitoring provides 24/7 real-time data on temperature, door openings, and system health. It sends instant alerts via text or email if any parameter deviates, allowing for immediate intervention before a temperature excursion occurs. This is essential for both proactive maintenance and regulatory compliance.
The Pitfall of Ignoring Total Cost
Focusing solely on the initial purchase price is a common mistake. The true cost includes energy consumption, potential maintenance, and—most importantly—the immense financial and scientific cost of a lost batch. Investing in a unit with higher reliability, better insulation, and robust backup systems provides a far greater return by minimizing the risk of failure.
Making the Right Choice for Your Goal
Your specific operational needs should dictate which features you prioritize.
- If your primary focus is large-scale, centralized storage: Prioritize maximum storage capacity, energy efficiency, and a robust LN2 or CO2 backup system.
- If your primary focus is a smaller clinic or research lab: Focus on a compact footprint, a highly modular interior for organizing diverse samples, and integrated cloud-based monitoring for peace of mind.
- If your primary focus is operational flexibility and future-proofing: Select a unit with a wide temperature setpoint range and excellent portability to adapt to changing needs.
Ultimately, the right freezer is an investment in the absolute integrity and security of your critical work.
Summary Table:
| Feature Category | Key Feature | Why It Matters |
|---|---|---|
| Core Functionality | Consistent -80°C Maintenance | Preserves fragile mRNA vaccine integrity by halting degradation. |
| Core Functionality | Wide Temperature Range (-50°C to -86°C) | Provides flexibility for storing other biologics and future formulations. |
| Operational Efficiency | High Storage Capacity & Compact Footprint | Maximizes vial storage while minimizing valuable lab space usage. |
| Operational Efficiency | Modular, Adjustable Interiors | Enables organized storage, quick retrieval, and reduced temperature fluctuation. |
| Failure Prevention | Integrated Backup Cooling System (LN2/CO2) | Prevents catastrophic loss during primary compressor failure or power outage. |
| Failure Prevention | Cloud-Based Temperature Monitoring | Provides 24/7 real-time alerts for immediate intervention and compliance. |
Secure Your Critical mRNA Vaccines with Confidence
Protecting your invaluable biological assets requires equipment you can trust. KINTEK specializes in providing reliable lab equipment and consumables, including ultra-low temperature freezers designed for the precise demands of vaccine storage. Our solutions ensure unwavering temperature integrity, operational efficiency, and robust failure prevention to safeguard your work.
Let us help you build a comprehensive storage system that meets your specific needs. Contact our experts today to discuss your requirements and find the perfect ULT freezer for your laboratory.
Visual Guide
Related Products
- 58L Precision Laboratory Ultra Low Temperature Upright Freezer for Critical Sample Storage
- 508L Advanced Vertical Ultra Low Temperature Freezer for Critical Laboratory Storage
- 408L Advanced Vertical Laboratory Ultra Low Temperature Freezer for Critical Research Material Preservation
- 708L Ultra Low Temperature Freezer High Performance Laboratory Freezer
- 208L Advanced Precision Laboratory Ultra Low Temperature Freezer for Cold Storage
People Also Ask
- What is the temperature control capability of ultra-low freezers? Precise Stability Down to -86°C
- What temperature ranges are typically associated with ultra-low temperature freezers? Preserve Samples from -40°C to -86°C
- What is the role of an ultra-low temperature (ULT) freezer in the freeze-thaw synthesis of hydrogel nanocomposites?
- What features do ultra-low temperature freezers typically include? Ensuring Absolute Sample Security
- How do ultra-low temperature freezers work? Unlocking the Secrets of -86°C Sample Preservation



















