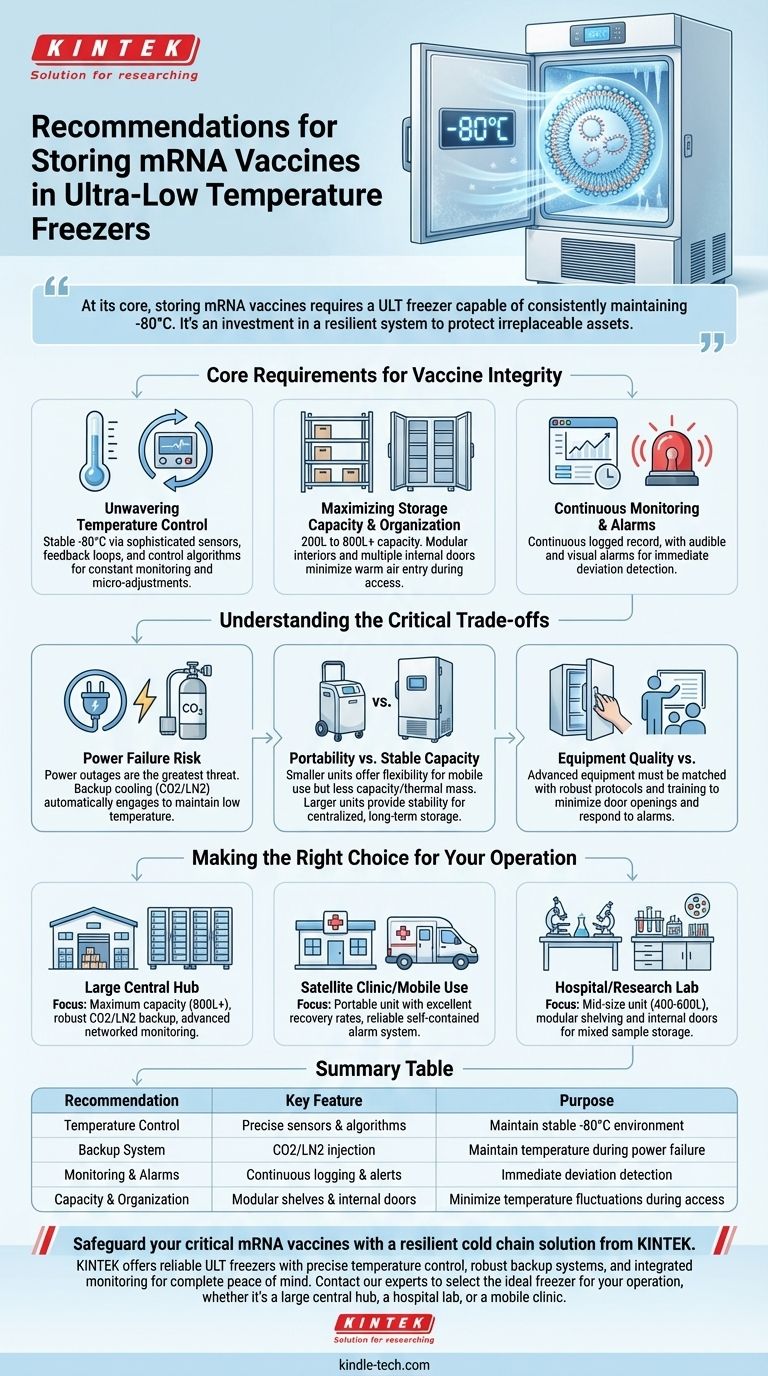
At its core, storing mRNA vaccines requires an ultra-low temperature (ULT) freezer capable of consistently maintaining -80°C. Key recommendations focus on selecting a unit with robust temperature control, a comprehensive backup system, integrated monitoring with alarms, and sufficient, well-organized capacity to minimize temperature fluctuations during access.
The challenge of storing mRNA vaccines is not merely achieving a low temperature, but ensuring absolute temperature stability. Your choice of a ULT freezer is an investment in a resilient system designed to protect irreplaceable assets from thermal degradation, power failure, and human error.

Core Requirements for Vaccine Integrity
Properly storing mRNA vaccines is fundamental to their efficacy. The freezer is not just a piece of equipment; it's the primary safeguard for the vaccine's delicate lipid nanoparticle structure.
Unwavering Temperature Control
The foundational requirement is the ability to maintain a stable internal environment, typically at or below -80°C. This is not a "set and forget" feature.
High-quality ULT freezers achieve this through a sophisticated system of sensors, feedback loops, and control algorithms. These systems constantly monitor the internal temperature and make micro-adjustments to the cooling cycle to prevent deviations that could compromise the vaccines.
Maximizing Storage Capacity and Organization
ULT freezers typically range in capacity from 200 to over 800 liters. However, raw volume is only part of the equation.
Effective organization is critical for temperature stability. Look for models with modular interiors, such as adjustable shelves and racks. Most importantly, seek out units with multiple internal doors. Each time the main door is opened, warm air enters. Internal doors ensure that only the specific shelf being accessed is exposed, protecting the rest of the inventory from temperature fluctuations.
Continuous Monitoring and Alarms
An integrated temperature monitoring system is non-negotiable. This system should provide a continuous, logged record of the freezer's temperature history, which is essential for compliance and quality control.
The system must also include audible and visual alarms that trigger immediately if the temperature deviates from the setpoint. This provides the crucial window of time needed to implement emergency protocols and prevent catastrophic loss.
Understanding the Critical Trade-offs
Selecting the right ULT freezer involves balancing competing priorities. Understanding these trade-offs is key to making an informed decision that aligns with your operational reality.
The Inevitable Risk of Power Failure
The single greatest threat to your vaccine inventory is a power outage. A standard ULT freezer, even with excellent insulation, will begin to warm up immediately.
A backup cooling system is the only reliable mitigation. These systems, often using liquid carbon dioxide (CO2) or liquid nitrogen (LN2), automatically engage during a power failure to inject cryogen and maintain the required low temperature for an extended period. This is an essential redundancy.
Portability vs. Stable Capacity
Smaller, more portable ULT freezers offer flexibility for mobile clinics or facilities with changing needs. However, this portability often comes at the cost of storage capacity and thermal mass.
Larger freezers are less mobile but provide greater storage density and are often more thermally stable due to their size. They are better suited for centralized, long-term storage hubs where the primary goal is maximizing inventory in a secure location.
Equipment Quality vs. Human Protocol
Even the most advanced freezer can be undermined by poor operational protocol. Frequent or prolonged door openings are a primary cause of temperature instability.
Your investment in hardware must be matched by an investment in training. Staff must understand the importance of minimizing door access, working quickly, and responding immediately to alarms. A robust freezer is only as effective as the protocols governing its use.
Making the Right Choice for Your Operation
Your specific storage environment and operational goals should dictate your final choice.
- If your primary focus is a large, central storage hub: Prioritize maximum capacity (800L+), a robust CO2/LN2 backup system, and advanced, networked temperature monitoring.
- If your primary focus is a satellite clinic or mobile use: Opt for a smaller, potentially portable unit with excellent temperature recovery rates and a reliable, self-contained alarm system.
- If your primary focus is a hospital or research lab with mixed storage needs: Select a mid-size unit (400-600L) with modular shelving and inner doors to accommodate various sample types alongside vaccines, and ensure it has a dependable backup system.
Ultimately, safeguarding mRNA vaccines hinges on creating a resilient cold chain environment built on precise control, constant monitoring, and robust contingency planning.
Summary Table:
| Recommendation | Key Feature | Purpose |
|---|---|---|
| Temperature Control | Precise sensors & algorithms | Maintain stable -80°C environment |
| Backup System | CO2/LN2 injection | Maintain temperature during power failure |
| Monitoring & Alarms | Continuous logging & alerts | Immediate deviation detection |
| Capacity & Organization | Modular shelves & internal doors | Minimize temperature fluctuations during access |
Safeguard your critical mRNA vaccines with a resilient cold chain solution from KINTEK.
Choosing the right ultra-low temperature freezer is a critical investment in protecting your valuable vaccines from thermal degradation and power failure. KINTEK specializes in lab equipment, offering reliable ULT freezers with the precise temperature control, robust backup systems, and integrated monitoring you need for complete peace of mind.
Let our experts help you select the ideal freezer for your operation, whether it's a large central hub, a hospital lab, or a mobile clinic. Contact KINTEK today to ensure your vaccine storage is secure, stable, and compliant.
Visual Guide
Related Products
- 408L Advanced Vertical Laboratory Ultra Low Temperature Freezer for Critical Research Material Preservation
- 108L Vertical Ultra Low Temperature ULT Freezer
- 508L Advanced Vertical Ultra Low Temperature Freezer for Critical Laboratory Storage
- 708L Ultra Low Temperature Freezer High Performance Laboratory Freezer
- 208L Advanced Precision Laboratory Ultra Low Temperature Freezer for Cold Storage
People Also Ask
- What is the purpose of ultra-low temperature (ULT) freezers? Preserve Critical Biological Samples
- How is temperature controlled in ultra low temperature freezers? A Guide to Stable -80°C Storage
- Why are ULT freezers considered vital equipment in labs? Ensuring Uncompromised Sample Integrity for Critical Research
- What are ultra-low temperature freezers designed for? Preserving Your Most Valuable Biological Samples
- What temperature ranges are typically associated with ultra-low temperature freezers? Preserve Samples from -40°C to -86°C



















