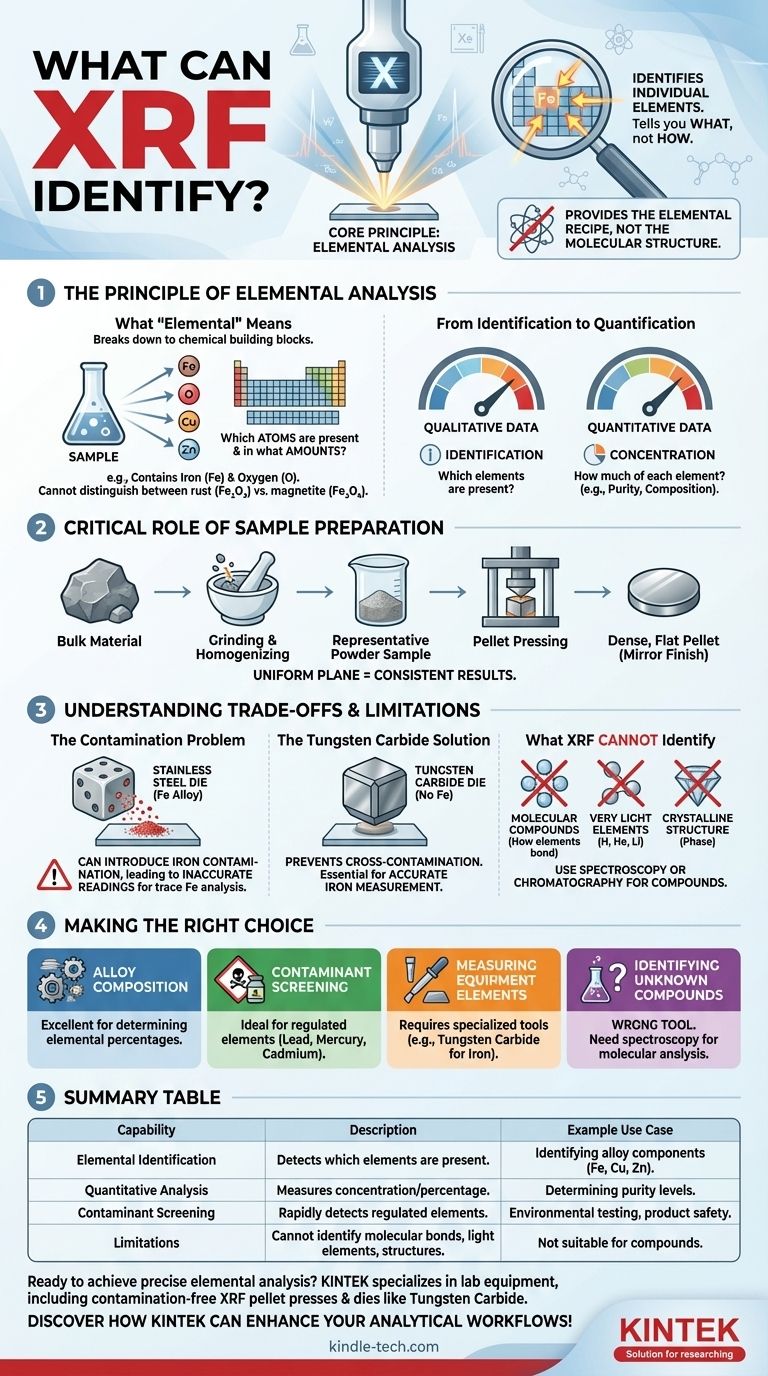
At its core, X-ray fluorescence (XRF) is a powerful technique that identifies the individual elements present within a material. It serves as an elemental analysis tool, capable of both detecting the presence of elements from the periodic table and quantifying their relative concentrations in a sample.
The crucial takeaway is that XRF tells you what a material is made of at the elemental level (e.g., iron, copper, zinc), but not how those elements are chemically bonded. It provides the elemental recipe, not the molecular structure.

The Principle of Elemental Analysis
What "Elemental" Means
XRF analysis breaks down a substance to its most basic chemical building blocks. It answers the question, "Which atoms from the periodic table are in this object, and in what amounts?"
For example, it can tell you that a sample contains iron and oxygen. However, it cannot, by itself, distinguish between different iron oxides like rust (Fe₂O₃) and magnetite (Fe₃O₄), as the chemical structure is beyond its scope.
From Identification to Quantification
The technique serves two primary functions. First, it provides qualitative data by identifying which elements are present.
Second, and often more importantly, it offers quantitative data, measuring the concentration or relative percentage of each identified element. This makes it invaluable for determining the exact composition of metal alloys or the purity of a substance.
The Critical Role of Sample Preparation
Creating a Representative Sample
To get an accurate reading of a bulk material, a representative sample must be prepared. This often involves grinding a fragment of the material into a fine, homogenous powder.
This homogenization is critical. It ensures that the small portion being analyzed has the exact same composition as the larger object it came from, eliminating variations that could skew the results.
The Pellet Pressing Process
This fine powder is then typically pressed into a small, dense disc or pellet. The pellet dies used for this process have a perfect mirror finish.
This flawless surface is not for aesthetics; it ensures the X-ray beam interacts with a completely flat and uniform plane, which is essential for achieving consistent and repeatable measurements between different samples.
Understanding the Trade-offs and Limitations
The Problem of Contamination
The tools used in the analysis can sometimes interfere with the results. For example, standard XRF pellet dies are made from hardened stainless steel.
Since steel is an iron alloy, using these dies to prepare a sample where you need to measure trace amounts of iron can introduce contamination. The die itself can shed microscopic iron particles into the sample, leading to an inaccurate, artificially high reading.
The Tungsten Carbide Solution
To overcome this specific problem, analysts use pellet dies with pressing faces made of a different material, such as Tungsten Carbide.
Because Tungsten Carbide contains no iron, it prevents cross-contamination and allows for the accurate measurement of iron within the sample. This highlights the importance of choosing the right equipment for the specific element being studied.
What XRF Cannot Identify
It is crucial to recognize the limits of XRF. The technique is not suitable for identifying:
- Molecular compounds or how elements are bonded.
- Very light elements like Hydrogen, Helium, or Lithium.
- The crystalline structure or phase of a material.
Making the Right Choice for Your Analysis
Understanding XRF's capabilities allows you to apply it effectively.
- If your primary focus is alloy composition: XRF is an excellent choice for quickly and accurately determining the elemental percentages in a metal sample.
- If your primary focus is screening for contaminants: The technique is ideal for rapidly detecting the presence of specific regulated elements, such as lead, mercury, or cadmium.
- If your primary focus is measuring an element also found in your equipment: You must use specialized tools, like Tungsten Carbide dies for iron analysis, to guarantee accurate results.
- If your primary focus is identifying an unknown chemical compound: XRF is the wrong tool; you would need a technique that analyzes molecular structure, such as spectroscopy or chromatography.
By knowing both what XRF can and cannot do, you can confidently leverage it for precise and reliable elemental insights.
Summary Table:
| Capability | Description | Example Use Case |
|---|---|---|
| Elemental Identification | Detects which elements are present in a sample. | Identifying alloy components like iron, copper, or zinc. |
| Quantitative Analysis | Measures the concentration or percentage of each element. | Determining purity levels in metal alloys or minerals. |
| Contaminant Screening | Rapidly detects regulated elements (e.g., lead, cadmium). | Environmental testing or product safety compliance. |
| Limitations | Cannot identify molecular bonds, light elements (H, He, Li), or crystalline structures. | Not suitable for compound identification—use spectroscopy instead. |
Ready to achieve precise elemental analysis in your lab? KINTEK specializes in lab equipment and consumables, including XRF pellet presses and dies made from contamination-free materials like Tungsten Carbide. Whether you're analyzing alloys, screening for contaminants, or need reliable sample preparation tools, our solutions ensure accurate and repeatable results. Contact us today to discuss your laboratory needs and discover how KINTEK can enhance your analytical workflows!
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are benefits of hydraulic press? High Force, Precision Control, and Cost-Effectiveness
- How are laboratory hydraulic presses applied in neural implant manufacturing? Precision Tools for Neural Electrodes
- How does a laboratory hydraulic press contribute to iodo-vanadate-lead ceramics? Optimize Green Body Preparation
- How high pressure is created in a lab? Master Safe and Precise Pressure Generation
- What is the maximum curing time required in a compression Moulding process? Find Your Optimal Cure Time for Perfect Parts
- Why the potassium bromide used to make the KBr pellet must be dry? Avoid Costly Errors in IR Spectroscopy
- What is the role of laboratory hydraulic presses or CIP in LFP solid-state battery assembly? Expert Insights
- Why is a uniaxial hydraulic press used for LLZTO powder? Achieving High Green Density for Ceramic Success



















