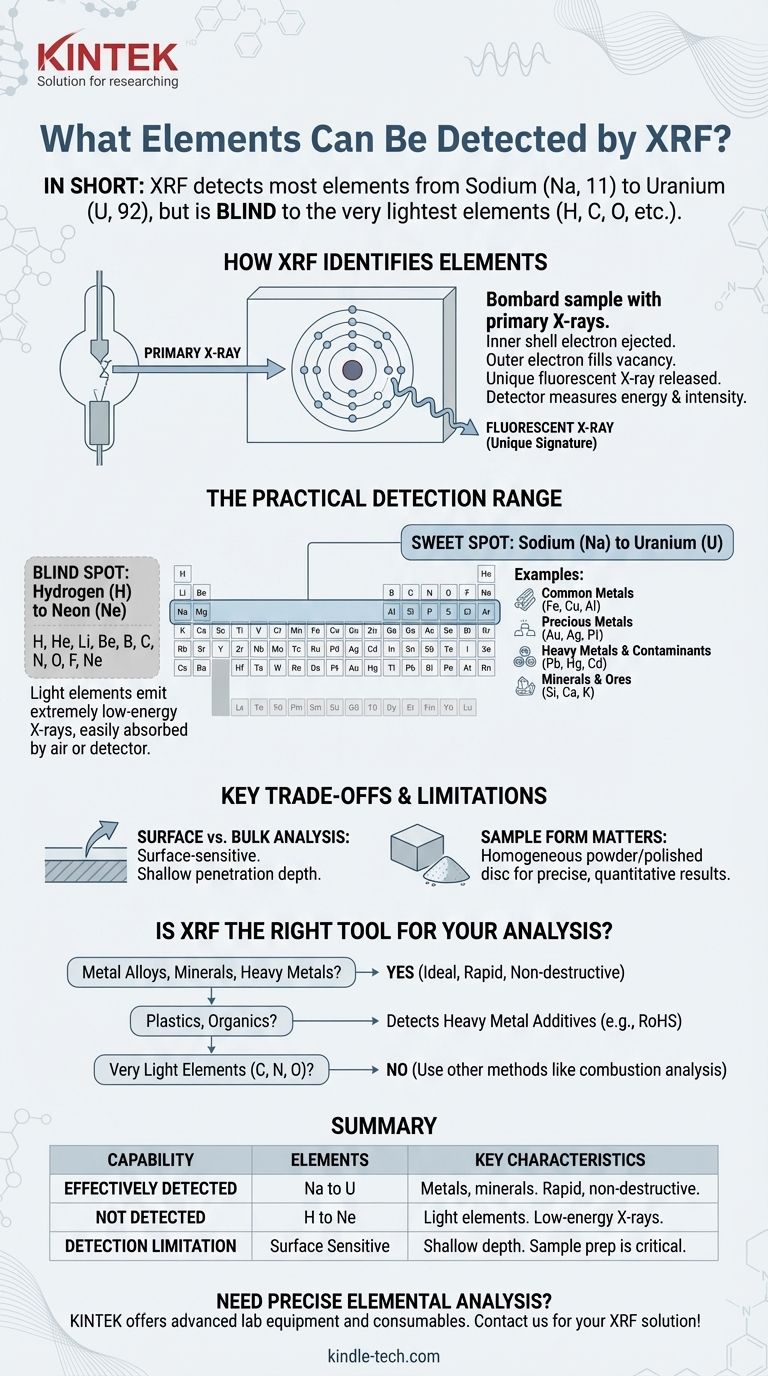
In short, X-ray fluorescence (XRF) can detect most elements on the periodic table, typically ranging from Sodium (Na, atomic number 11) all the way up to Uranium (U, atomic number 92). The technology is a powerful tool for identifying and quantifying the elemental composition of materials. However, it is fundamentally unable to detect the very lightest elements, such as hydrogen, carbon, or oxygen, with standard equipment.
XRF is the go-to method for rapid elemental analysis of metals, minerals, and heavy elements. Its primary limitation is a "blind spot" for elements lighter than Sodium, which is a critical factor when deciding if it's the right tool for your specific analytical needs.

How XRF Identifies Elements
To understand which elements XRF can detect, it's essential to understand its basic operating principle. The process isn't magic; it's governed by the physics of atoms.
The Fundamental Principle
An XRF instrument bombards a sample with high-energy primary X-rays. This energy can knock an electron out of an inner atomic shell of an atom in the sample. This creates an unstable vacancy, which is immediately filled by an electron from a higher-energy outer shell. As the electron drops into the lower energy state, it releases a secondary X-ray—a process called fluorescence.
Why Every Element Has a Unique Signature
The energy of this fluorescent X-ray is unique to the element from which it was emitted. A copper atom will release a fluorescent X-ray with a different energy than an iron atom. The XRF detector measures both the energy and intensity of all the emitted secondary X-rays to identify and quantify the elements present in the sample.
The Challenge with Light Elements
Very light elements, such as Carbon (C), Nitrogen (N), and Oxygen (O), have very few electrons. The fluorescent X-rays they emit are extremely low-energy. These weak X-rays are easily absorbed by the surrounding air or even the instrument's detector window before they can be measured. This physical limitation is why standard XRF cannot detect them.
The Practical Detection Range of XRF
While the theoretical range is broad, the practical application has a clear "sweet spot" and well-defined boundaries.
The Sweet Spot: Sodium to Uranium
For most common benchtop and handheld XRF analyzers, the effective range begins at Sodium (Na) or Magnesium (Mg) and extends to Uranium (U). This covers a vast and commercially important list of elements, including:
- Common Metals: Iron (Fe), Copper (Cu), Nickel (Ni), Aluminum (Al), Titanium (Ti)
- Precious Metals: Gold (Au), Silver (Ag), Platinum (Pt), Palladium (Pd)
- Heavy Metals & Contaminants: Lead (Pb), Mercury (Hg), Cadmium (Cd), Arsenic (As)
- Minerals & Ores: Silicon (Si), Calcium (Ca), Potassium (K), Sulfur (S)
Elements Beyond XRF's Reach
Standard XRF systems are effectively "blind" to the first 10 elements on the periodic table. These include:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
Understanding the Trade-offs and Limitations
Choosing an analytical method requires understanding its limitations. XRF is powerful but is not universally applicable.
Surface vs. Bulk Analysis
XRF is fundamentally a surface-sensitive technique. The primary X-rays only penetrate a shallow depth into the material, typically from a few micrometers to several millimeters depending on the sample's density. The analysis, therefore, represents the composition of the surface, which may not be representative of the bulk material if the sample is not uniform.
The Importance of Sample Form
The accuracy of XRF results is highly dependent on the sample's form. While you can analyze solid objects like scrap metal directly, this often provides qualitative data. For precise, quantitative results, materials are often homogenized into a fine powder or prepared as a flat, polished solid disc. This ensures the surface being measured is a true representation of the entire sample.
Not All XRF Instruments are Equal
Advanced laboratory XRF systems that use a vacuum or a helium gas purge can improve the detection of lighter elements like Magnesium (Mg), Aluminum (Al), and Silicon (Si). However, even these specialized systems cannot overcome the physical barrier to detecting elements like carbon or oxygen.
Is XRF the Right Tool for Your Analysis?
Your choice depends entirely on the elements you need to measure.
- If your primary focus is analyzing metal alloys, minerals, soils, or testing for heavy metals in consumer products: XRF is an ideal, rapid, and often non-destructive method for this purpose.
- If your primary focus is identifying plastics or analyzing organic materials: XRF is useful for detecting restricted heavy metal additives (like in RoHS testing) but cannot determine the base polymer's composition (carbon, hydrogen, etc.).
- If your primary focus is measuring very light elements like carbon, nitrogen, or oxygen: You must use a different analytical technique, such as combustion analysis or Leco analysis, as XRF cannot detect these elements.
Ultimately, selecting the correct analytical instrument requires matching its capabilities to the specific elemental questions you need to answer.
Summary Table:
| XRF Detection Capability | Elements | Key Characteristics |
|---|---|---|
| Effectively Detected | Sodium (Na) to Uranium (U) | Ideal for metals, minerals, heavy metals, and precious metals. Provides rapid, non-destructive analysis. |
| Not Detected (Standard XRF) | Hydrogen (H) to Neon (Ne) | Light elements emit low-energy X-rays absorbed by air. Includes carbon, nitrogen, and oxygen. |
| Detection Limitation | Surface-sensitive technique | Analysis depth is shallow. Sample preparation (powder, polished disc) is critical for accurate quantitative results. |
Need precise elemental analysis for your materials? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Whether you're analyzing metal alloys, minerals, or screening for contaminants, our XRF solutions deliver rapid, reliable results for elements from Sodium to Uranium. Contact us today to find the perfect analytical tool for your laboratory's specific requirements!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- KF Ultra-High Vacuum Observation Window 304 Stainless Steel Flange High Borosilicate Glass Sight Glass
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- Why is Vacuum Drying Equipment used for Na3SbS4-xSex? Ensure High-Purity Sulfide Electrolyte Processing
- What is the main product of slow pyrolysis? Maximize Biochar Yield for Soil & Carbon Sequestration
- Why argon gas is used in sputtering? Achieve Pure, Cost-Effective Thin Film Deposition
- What are alternatives to lab-grown diamonds? Compare Natural Diamonds, Moissanite & More
- What affects sputtering yield? Master the Physics for Maximum Deposition Efficiency
- What is the difference between thermal and catalytic pyrolysis? Maximize Yield vs. Improve Oil Quality
- What is sputtering technology? A Guide to Precision Thin Film Deposition
- What are the advantages and limitations for heat treatment process? Mastering Material Strength and Surface Integrity















