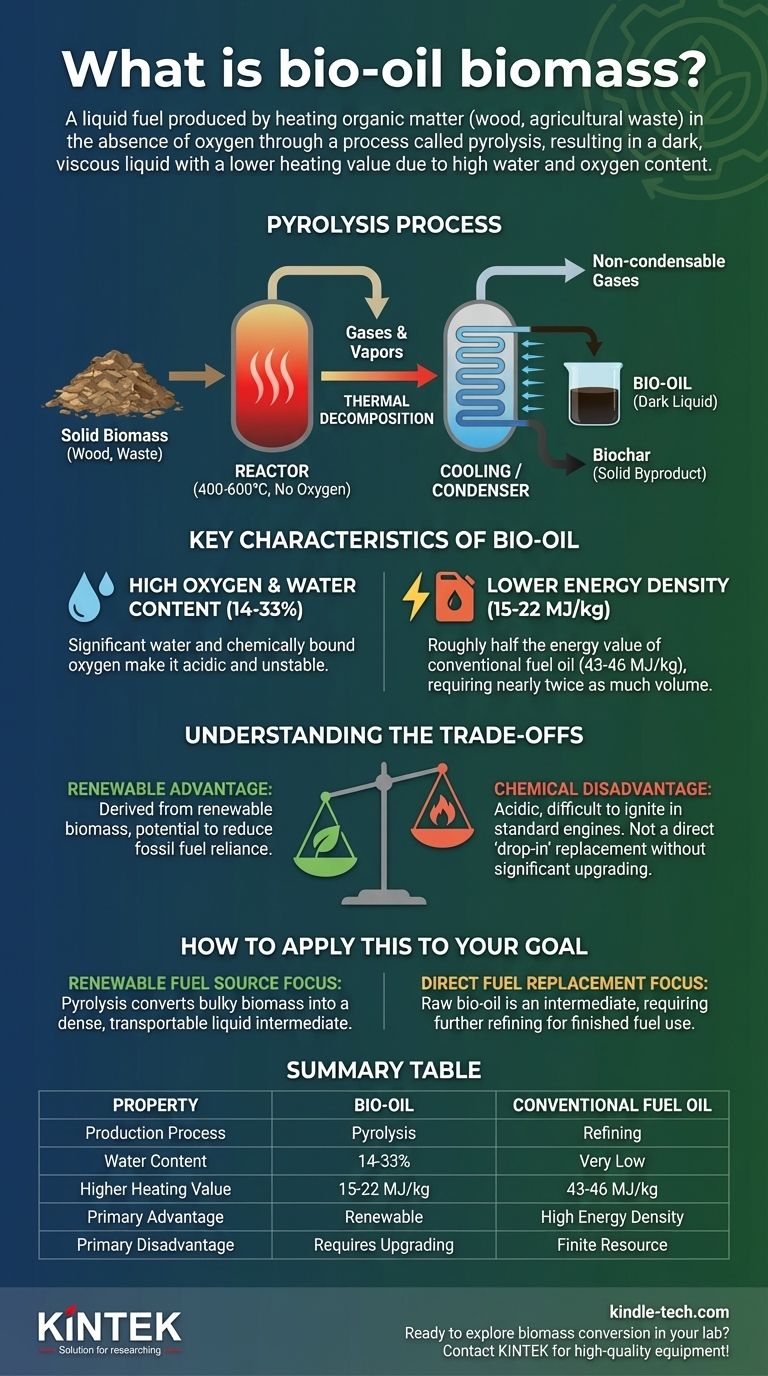
Bio-oil from biomass is a liquid fuel produced by heating organic matter, such as wood or agricultural waste, to high temperatures in an environment without oxygen. This process, known as pyrolysis, breaks down the solid biomass into gases that are then cooled and condensed into a dark, viscous liquid. This resulting bio-oil has a much lower heating value than conventional fuel oil primarily due to its significant water and oxygen content.
While bio-oil represents a direct method for converting solid biomass into a liquid energy carrier, its inherent chemical properties—high water and oxygen content—make it fundamentally different and less energy-dense than traditional fossil fuels, requiring further refinement for most applications.
How Bio-Oil is Produced: The Pyrolysis Process
The conversion of solid biomass into liquid bio-oil is achieved through a thermochemical process called pyrolysis. Understanding this process is key to understanding the final product's characteristics.
Heating Without Oxygen
Pyrolysis involves heating biomass rapidly to high temperatures, typically between 400-600°C. Crucially, this is done in the complete absence of oxygen.
This lack of oxygen prevents the biomass from combusting (burning) and instead causes it to thermally decompose into smaller molecules.
From Solid to Liquid
As the biomass breaks down, it forms a mixture of gases and vapors. These hot gases are then rapidly cooled, or "quenched."
This cooling process condenses the vapors into a liquid, which is the raw bio-oil. Along with the liquid, this process also produces non-condensable gases and a solid carbon-rich byproduct called biochar.
Key Characteristics of Bio-Oil
Bio-oil is often called "pyrolysis oil," and its properties differ significantly from petroleum-based fuels. These differences are a direct result of its biomass origin and production method.
High Oxygen and Water Content
Unlike conventional fuels, bio-oil contains a large amount of oxygen bound within its chemical structure.
It also has a very high water content, typically ranging from 14% to 33% by weight. This water is not easily removed by simple methods like distillation and can cause the oil to separate into different phases.
Lower Energy Density
The presence of oxygen and water dramatically reduces the energy content of bio-oil.
Its higher heating value is typically 15–22 MJ/kg. This is roughly half the value of conventional fuel oil, which sits in the 43–46 MJ/kg range. You need almost twice as much bio-oil to produce the same amount of energy.
Understanding the Trade-offs
Bio-oil presents a classic trade-off between renewability and performance. Its viability depends entirely on the intended application and willingness to invest in further processing.
The Renewable Advantage
The primary benefit of bio-oil is its origin. It is derived from renewable biomass, offering a potential pathway to reduce reliance on finite fossil fuels and create a more circular carbon economy.
The Chemical Disadvantage
The high oxygen and water content make raw bio-oil acidic, unstable, and difficult to ignite in standard engines. It cannot be used as a "drop-in" replacement for gasoline or diesel without significant and often costly upgrading to remove oxygen and improve its properties.
How to Apply This to Your Goal
Your perspective on bio-oil will depend entirely on your objective, as it is not a one-size-fits-all energy solution.
- If your primary focus is creating a renewable fuel source: Pyrolysis is a viable technology for converting bulky solid biomass into a dense, transportable liquid intermediate.
- If your primary focus is finding a direct replacement for conventional fuels: Raw bio-oil is unsuitable and must be viewed as a starting point for further refining, not as a finished fuel.
Ultimately, recognizing bio-oil as a chemically distinct intermediate is the key to evaluating its true potential in the renewable energy landscape.
Summary Table:
| Property | Bio-Oil | Conventional Fuel Oil |
|---|---|---|
| Production Process | Pyrolysis (heating without oxygen) | Refining of crude oil |
| Water Content | 14-33% | Very Low |
| Higher Heating Value | 15-22 MJ/kg | 43-46 MJ/kg |
| Primary Advantage | Renewable, from biomass | High energy density |
| Primary Disadvantage | Requires upgrading for engine use | Finite resource, fossil fuel |
Ready to explore biomass conversion in your lab? KINTEK specializes in high-quality lab equipment for pyrolysis research and analysis. Whether you're developing new bio-oil upgrading processes or characterizing biomass feedstocks, our tools can help you achieve precise and reliable results. Contact our experts today to discuss how we can support your renewable energy projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- High Performance Laboratory Freeze Dryer for Research and Development
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- Are single stage furnaces more reliable? Discover the truth about HVAC durability vs. comfort.
- Why must hydrogen-charged 316L stainless steel samples be stored in liquid nitrogen? Ensure Accurate TDS Analysis
- What is the required temperature for ash content determination? Achieve Accurate Mineral Analysis in Your Lab
- What is sputter ceramic film? A High-Tech Solution for Superior Heat Rejection & Clarity
- What is the purpose of using heat treatment? Tailor Material Properties for Superior Performance
- How does a laboratory magnetic stirrer contribute to pre-mixing? Master Your Photocatalytic Reaction Baselines
- What are the advantages and disadvantages of the sintering process? Achieve Strong, Complex Parts with High-Temp Materials
- What are the 3 factors that affect the rate of heat transfer by conduction? Master Thermal Control for Your Lab Equipment



















