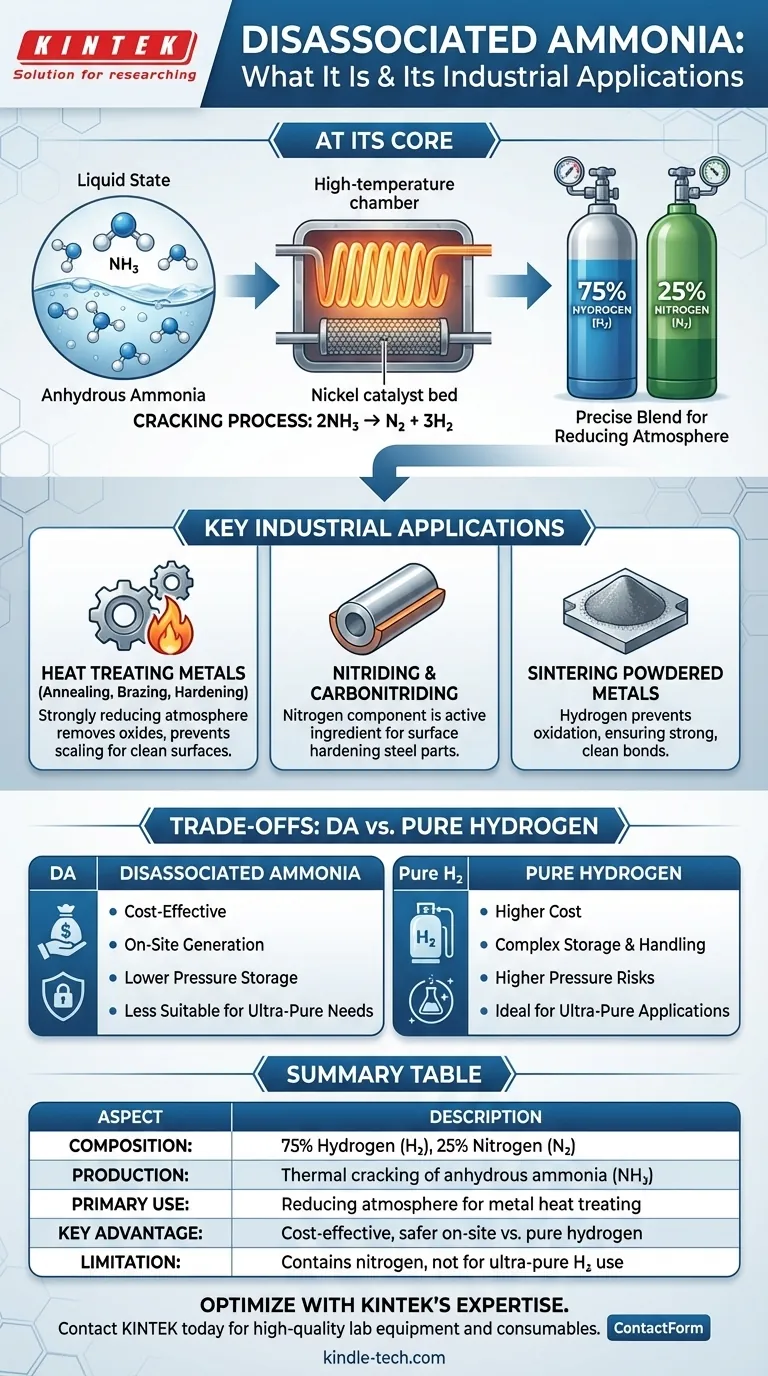
At its core, disassociated ammonia is a specific gas mixture produced by "cracking" or decomposing anhydrous ammonia (NH₃) using heat and a catalyst. The resulting gas is a precisely controlled blend of 75% hydrogen (H₂) and 25% nitrogen (N₂) by volume. This mixture is not a unique chemical compound but rather a highly practical and cost-effective source of a hydrogen-rich atmosphere for industrial applications.
The central concept to grasp is that disassociated ammonia is used as a convenient and economical on-site source of hydrogen and nitrogen. It allows facilities to leverage the relative safety and low cost of storing liquid ammonia while generating a powerful reducing gas atmosphere when and where it is needed.

The Dissociation Process: From Liquid to Gas
Understanding how this gas mixture is created is key to understanding its properties and applications. The process is a straightforward chemical reaction carried out inside a dedicated piece of equipment.
The Starting Point: Anhydrous Ammonia
The process begins with anhydrous ammonia (NH₃), a compound that is liquid under moderate pressure at room temperature. This makes it significantly easier, safer, and cheaper to transport and store in bulk compared to pure hydrogen gas.
The Core Reaction: Thermal Cracking
The liquid ammonia is vaporized and then passed through a high-temperature chamber (typically 1750-1850°F or 950-1010°C) containing a nickel catalyst. This combination of heat and catalytic action breaks the ammonia molecules apart.
The chemical reaction is simple and highly efficient: 2NH₃ → N₂ + 3H₂.
The Final Product: A 75/25 Blend
This reaction shows that for every two molecules of ammonia that are broken down, one molecule of nitrogen and three molecules of hydrogen are created. This is what results in the consistent final mixture of 25% nitrogen and 75% hydrogen by volume. The equipment used for this is often called an ammonia dissociator or cracked ammonia generator.
Key Applications in Industry
Disassociated ammonia is not just a chemical curiosity; it is a workhorse in metallurgical and chemical processes where atmospheric control is critical.
Heat Treating Metals
This is the most common application. When heating metals like steel for processes such as annealing, hardening, or brazing, the oxygen in the air can cause destructive scaling and oxidation. A disassociated ammonia atmosphere is strongly reducing (due to the hydrogen), meaning it actively removes oxides and prevents new ones from forming, resulting in a clean, bright metal surface.
Nitriding and Carbonitriding
In these specialized surface-hardening processes for steel, the nitrogen component is not just an inert gas—it's the active ingredient. The nitrogen from the dissociated ammonia reacts with the surface of the steel part to form extremely hard iron nitrides, creating a wear-resistant case.
Sintering Powdered Metals
Sintering involves heating compacted metal powders to just below their melting point to fuse them into a solid object. The hydrogen-rich atmosphere of disassociated ammonia prevents oxidation of the fine metal particles and helps create strong, clean bonds between them.
Understanding the Trade-offs: DA vs. Pure Hydrogen
The decision to use disassociated ammonia almost always comes down to a practical comparison with its primary alternative, pure hydrogen.
The Decisive Factor: Cost
Generating a hydrogen-rich atmosphere from ammonia is significantly less expensive than purchasing and storing purified bulk hydrogen (either as a high-pressure gas or a cryogenic liquid). The low cost and simple logistics of storing liquid ammonia provide a major economic advantage.
Safety and On-Site Generation
While anhydrous ammonia is toxic and requires careful handling, it is stored at much lower pressures than compressed hydrogen gas. The ability to generate the atmosphere on-demand from a stable liquid precursor is often seen as a logistical and safety advantage over handling highly flammable, high-pressure hydrogen cylinders or tanks.
The Purity Limitation
The most significant trade-off is purity. The 25% nitrogen content makes disassociated ammonia unsuitable for applications that require ultra-pure hydrogen, such as in the semiconductor or food industries. In most metallurgical work, however, the nitrogen is inert and harmless, or in the case of nitriding, actively beneficial.
Making the Right Choice for Your Process
Choosing the right industrial atmosphere depends entirely on the technical requirements of your process and your operational budget.
- If your primary focus is cost-effective metal heat treating (like annealing or brazing): Disassociated ammonia is an excellent choice, providing a high-quality reducing atmosphere without the expense and handling complexities of pure hydrogen.
- If your primary focus is surface hardening of steel parts: Disassociated ammonia is often the required source for the active nitrogen needed in nitriding and ferritic nitrocarburizing processes.
- If your primary focus is a process that is sensitive to nitrogen (e.g., semiconductor manufacturing or certain catalyst reactions): You must use a purified hydrogen source, as the nitrogen in disassociated ammonia would act as a critical contaminant.
Ultimately, understanding disassociated ammonia is about recognizing it as an engineered solution to a common industrial problem: the need for a safe, reliable, and economical source of hydrogen.
Summary Table:
| Aspect | Description |
|---|---|
| Composition | 75% Hydrogen (H₂), 25% Nitrogen (N₂) by volume |
| Production | Thermal cracking of anhydrous ammonia (NH₃) |
| Primary Use | Reducing atmosphere for metal heat treating (annealing, brazing) |
| Key Advantage | Cost-effective and safer on-site generation vs. pure hydrogen |
| Limitation | Contains nitrogen, unsuitable for ultra-pure H₂ applications |
Optimize your heat treating process with KINTEK's expertise.
Disassociated ammonia generators offer a safe, reliable, and economical solution for creating the perfect reducing atmosphere for your metal annealing, brazing, or sintering applications. KINTEK specializes in providing high-quality lab equipment and consumables to meet the precise needs of industrial laboratories.
Let our experts help you determine if a disassociated ammonia system is the right choice for your operation's efficiency and budget.
Contact KINTEK today to discuss your specific requirements!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- What are the functions of nitrogen (N2) in controlled furnace atmospheres? Achieve Superior Heat Treatment Results
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality



















