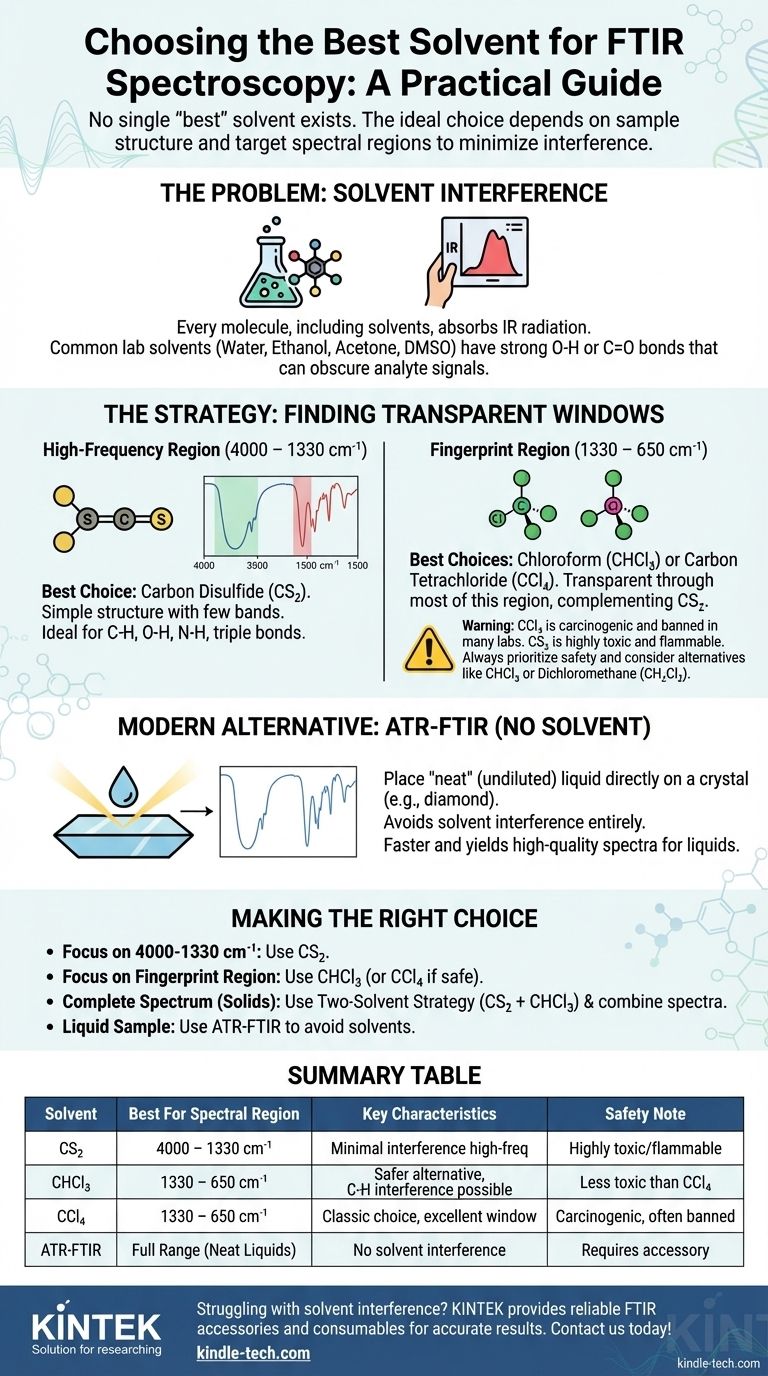
In short, there is no single "best" solvent for FTIR spectroscopy. The ideal choice is entirely dependent on the chemical structure of your sample and the specific spectral regions you need to analyze. The most common and effective approach involves using solvents like carbon disulfide (CS₂) and carbon tetrachloride (CCl₄) or chloroform (CHCl₃) because their own absorption bands are simple and predictable, leaving large "windows" of transparency to view your compound of interest.
The core challenge of solvent selection in FTIR is that every solvent absorbs infrared radiation to some degree. The strategy, therefore, is not to find a perfectly "invisible" solvent, but to choose one whose absorption bands do not overlap with the important vibrational bands of your analyte.

The Problem: Solvent Interference
Every molecule, including a solvent molecule, is composed of chemical bonds that vibrate when exposed to infrared radiation. These vibrations cause absorption bands in an IR spectrum.
The Ideal vs. The Reality
An ideal solvent would be "IR transparent," meaning it has no vibrations that absorb radiation in the mid-IR range (4000-400 cm⁻¹). No such solvent exists.
The goal is to select a solvent that interferes as little as possible. This typically means a small, simple molecule with few or no bonds corresponding to common functional groups, such as O-H, N-H, or C=O.
Why Common Lab Solvents Fail
Solvents like water, ethanol, acetone, and DMSO are generally poor choices for transmission FTIR. They contain O-H or C=O bonds, which absorb very strongly and create broad, intense peaks that can easily obscure the entire spectrum of the dissolved sample.
A Practical Guide to Common FTIR Solvents
The best practice often involves using a pair of solvents to piece together a full spectrum. One solvent is used for the high-frequency region, and another is used for the low-frequency "fingerprint" region.
For the High-Frequency Region (4000 – 1330 cm⁻¹)
Carbon Disulfide (CS₂) is the premier choice for this region.
Its simple, linear structure (S=C=S) means it only has a few absorption bands. It is largely transparent where C-H, O-H, N-H, and triple bond stretches appear, making it ideal for analyzing these critical functional groups. Its main interference is a strong band around 1535-1485 cm⁻¹.
For the Fingerprint Region (1330 – 400 cm⁻¹)
Carbon Tetrachloride (CCl₄) is the classic choice for this region.
It is a simple, symmetrical molecule that is transparent through most of the mid-IR range, but it has very strong absorptions below ~800 cm⁻¹. This makes it the perfect complement to CS₂, as its transparent "window" covers the region where CS₂ absorbs.
Modern & Safer Alternatives
Chloroform (CHCl₃) and Dichloromethane (CH₂Cl₂) are often used as more practical and less toxic alternatives to CCl₄.
They are better all-around solvents but have more C-H bonds, meaning they have more interfering peaks than CCl₄. However, they still offer large, useful windows and are a good compromise between spectral clarity and solvent utility. Chloroform, for instance, is a good choice for the fingerprint region but has C-H bands that will interfere around 3000 cm⁻¹ and 1200 cm⁻¹.
Understanding the Trade-offs
Choosing a solvent is a balancing act between spectral clarity, solubility of your sample, and safety.
The Two-Solvent Strategy
The most rigorous method for obtaining a full spectrum of a soluble compound is to run two separate experiments:
- Dissolve the sample in **carbon disulfide (CS₂) ** to get a clean view of the 4000 – 1330 cm⁻¹ region.
- Dissolve a second sample in chloroform (CHCl₃) or CCl₄ to get a clean view of the 1330 – 650 cm⁻¹ region.
You can then digitally combine the useful portions of both spectra to create one complete, interference-free spectrum.
The Critical Issue of Toxicity
Many of the "best" FTIR solvents are hazardous. Carbon tetrachloride is a known carcinogen and is banned in most modern laboratories. Carbon disulfide is highly toxic and extremely flammable.
Always consult a Safety Data Sheet (SDS) and use appropriate personal protective equipment (PPE), including working in a fume hood, when handling these chemicals. Safety often dictates using a slightly less "perfect" but safer solvent like chloroform or dichloromethane.
The Modern Alternative: No Solvent at All
For many liquid samples, the best solvent is no solvent. Attenuated Total Reflectance (ATR) is a modern sampling technique that has revolutionized routine FTIR analysis.
ATR-FTIR allows you to place a single drop of a "neat" (undiluted) liquid directly onto a crystal surface (often diamond). The IR beam interacts with the sample at the interface, producing a high-quality spectrum without any solvent interference. If your sample is a liquid and you have an ATR accessory, it is almost always faster, easier, and yields a cleaner spectrum than the traditional transmission method.
Making the Right Choice for Your Analysis
- If your primary focus is the C-H, N-H, O-H, or alkyne region (4000-1330 cm⁻¹): Your best choice is carbon disulfide (CS₂).
- If your primary focus is the fingerprint region (1330-650 cm⁻¹): Your best choice is chloroform (CHCl₃) or, if safety protocols permit, carbon tetrachloride (CCl₄).
- If you need a complete, publication-quality spectrum of a solid: Use the two-solvent strategy, combining a spectrum from CS₂ with one from CHCl₃.
- If your sample is a liquid and you want to avoid solvent interference entirely: Use an ATR-FTIR accessory to analyze the neat liquid directly.
By understanding that the goal is to find spectral windows, you can confidently select a solvent that reveals your sample's structure instead of obscuring it.
Summary Table:
| Solvent | Best For Spectral Region | Key Characteristics |
|---|---|---|
| Carbon Disulfide (CS₂) | 4000 – 1330 cm⁻¹ (C-H, O-H, N-H) | Minimal interference in high-frequency region; highly toxic/flammable |
| Chloroform (CHCl₃) | 1330 – 650 cm⁻¹ (Fingerprint region) | Safer alternative to CCl₄; good for fingerprint analysis |
| Carbon Tetrachloride (CCl₄) | 1330 – 650 cm⁻¹ (Fingerprint region) | Classic choice but carcinogenic; largely banned |
| ATR-FTIR (No Solvent) | Full range (Neat liquids) | Modern technique; avoids solvent interference entirely |
Struggling with solvent interference in your FTIR analysis? KINTEK specializes in lab equipment and consumables, providing reliable FTIR accessories and solvents tailored to your laboratory's needs. Whether you're optimizing spectral clarity or ensuring safety compliance, our expertise helps you achieve accurate, interference-free results. Contact us today to find the perfect solution for your FTIR challenges!
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Bomb Type Probe for Steelmaking Production Process
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
People Also Ask
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements
- What are the advantages and disadvantages of the ceramic core type copper sulfate reference electrode?
- How should a copper sulfate reference electrode be stored? A Guide to Short-Term & Long-Term Storage
- What precautions should be taken when handling and using a copper sulfate reference electrode? Ensure Accurate Electrochemical Measurements
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained



















