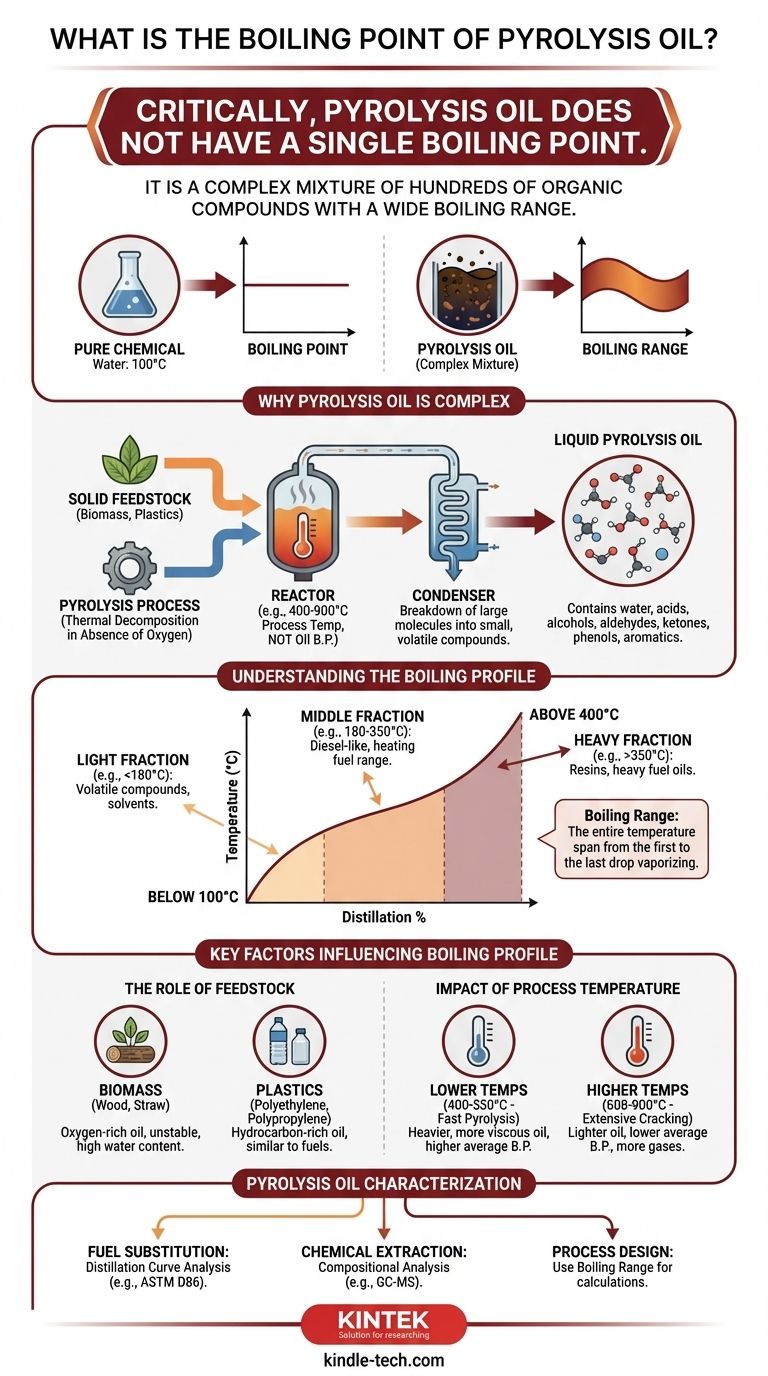
Critically, pyrolysis oil does not have a single boiling point. Instead, because it is a complex mixture of hundreds of different organic compounds, it has a wide boiling range. The temperatures mentioned in pyrolysis literature (400-900°C) refer to the temperature of the process used to create the oil, not the boiling point of the final liquid product.
The properties of pyrolysis oil, including its boiling characteristics, are not fixed. They are a direct result of the original feedstock (like wood or plastic) and the specific process conditions used, making it more akin to crude oil than a pure chemical compound.

Why Pyrolysis Oil is a Complex Mixture
To understand its properties, you must first understand its origin. Pyrolysis oil, also known as bio-oil or bio-crude, is not a substance that is refined; it is a substance that is created.
From Solid Feedstock to Liquid Fuel
Pyrolysis is the thermal decomposition of organic material in the absence of oxygen. This process breaks down large, complex molecules (like cellulose in wood or polymers in plastic) into a vast array of smaller, volatile molecules.
When these hot vapors are rapidly cooled, they condense into a liquid: pyrolysis oil. The liquid is essentially a snapshot of this chaotic chemical breakdown.
Hundreds of Different Compounds
The resulting oil is a complex mixture containing water, organic acids, alcohols, aldehydes, ketones, phenols, and larger aromatic and oxygenated compounds. Each of these individual chemicals has its own distinct boiling point.
The Crude Oil Analogy
The best way to think about pyrolysis oil is to compare it to fossil crude oil. No one asks for "the boiling point of crude oil." Instead, we separate crude oil via distillation into fractions with specific boiling ranges, such as gasoline, kerosene, and diesel. Pyrolysis oil must be viewed in the same way.
Understanding the Boiling Profile
Because it is a mixture, the boiling behavior of pyrolysis oil is described by a distillation curve, not a single point.
What is a Boiling Range?
When you heat pyrolysis oil, the most volatile compounds (those with the lowest boiling points) will vaporize first. As you continue to increase the temperature, heavier and more complex compounds will begin to boil.
This entire temperature span, from where the first drop of vapor boils to where the last drop of liquid boils, is the boiling range.
Typical Distillation Fractions
The boiling range can be very wide, often spanning from below 100°C to well over 400°C. For instance, a light fraction might be distilled below 180°C, while a heavy fraction might require temperatures exceeding 350°C. These fractions have vastly different properties and potential uses.
Key Factors Influencing the Boiling Profile
You cannot define the properties of a pyrolysis oil without knowing the context of its creation. The boiling profile is directly controlled by two primary factors.
The Role of Feedstock
The original material dictates the chemical makeup of the oil.
- Biomass (e.g., wood, straw): Tends to produce an oil rich in oxygenated compounds like acids, alcohols, and phenols. This often leads to instability and a high water content.
- Plastics (e.g., polyethylene, polypropylene): Tends to produce a non-oxygenated, hydrocarbon-rich oil more similar to traditional fuels, with distinct boiling fractions corresponding to gasoline or diesel ranges.
The Impact of Process Temperature
The temperature of the pyrolysis reactor has a profound effect on the final product.
- Lower Temperatures (e.g., 400-550°C): This "fast pyrolysis" favors the production of larger molecules, resulting in a heavier, more viscous oil with a higher average boiling point.
- Higher Temperatures (e.g., 600-900°C): This promotes more extensive "cracking," where larger molecules are broken down further. This results in a lighter oil with a lower average boiling point and a higher yield of non-condensable gases.
How to Approach Pyrolysis Oil Characterization
To work with pyrolysis oil effectively, you must analyze its specific properties for your intended application.
- If your primary focus is fuel substitution: You must analyze the oil's distillation curve (e.g., using ASTM D86 or a similar method) to see how its fractions align with fuels like diesel or heating oil.
- If your primary focus is chemical extraction: You need detailed compositional analysis (like GC-MS) to identify valuable compounds, as their individual boiling points will determine separation strategies.
- If your primary focus is process design: You must treat the oil as a multi-component mixture and use its boiling range, not a single point, for any heat exchange or distillation calculations.
Ultimately, understanding pyrolysis oil requires shifting your perspective from a single substance to a complex, variable mixture defined by its origin.
Summary Table:
| Key Factor | Impact on Boiling Profile |
|---|---|
| Feedstock Type | Biomass yields oxygenated compounds; plastics yield hydrocarbon fractions. |
| Process Temperature | Lower temps (400-550°C) create heavier oil; higher temps (600-900°C) create lighter oil. |
| Analogy | Similar to crude oil, it must be separated by distillation into fractions. |
Need precise analysis of your pyrolysis oil's properties? KINTEK specializes in lab equipment and consumables for detailed characterization, including distillation and compositional analysis. Our solutions help you optimize your process and identify valuable fuel or chemical fractions. Contact our experts today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Lab-Scale Vacuum Induction Melting Furnace
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
People Also Ask
- Where are ultra low temperature freezers commonly used? Essential for Labs, Hospitals, and Biotech
- How do industrial-grade constant temperature drying ovens ensure GO anti-corrosion coating performance?
- What are the disadvantages of metal iron? Key Limitations and Why We Use Alloys Instead
- What is the role of a laboratory oven in ZnO-Au nanocomposites? Achieve Precision Drying and Material Stability
- Is a filter press better than a clarifier? Choose the Right Tool for Your Separation Goal
- What is bio-oil biomass? A Liquid Fuel from Renewable Pyrolysis
- What is the range of RF sputtering? Expanding Your Thin Film Capabilities Beyond Metals
- What are the applications of melting temperature? Master Material Control for Joining, Casting & Alloying







