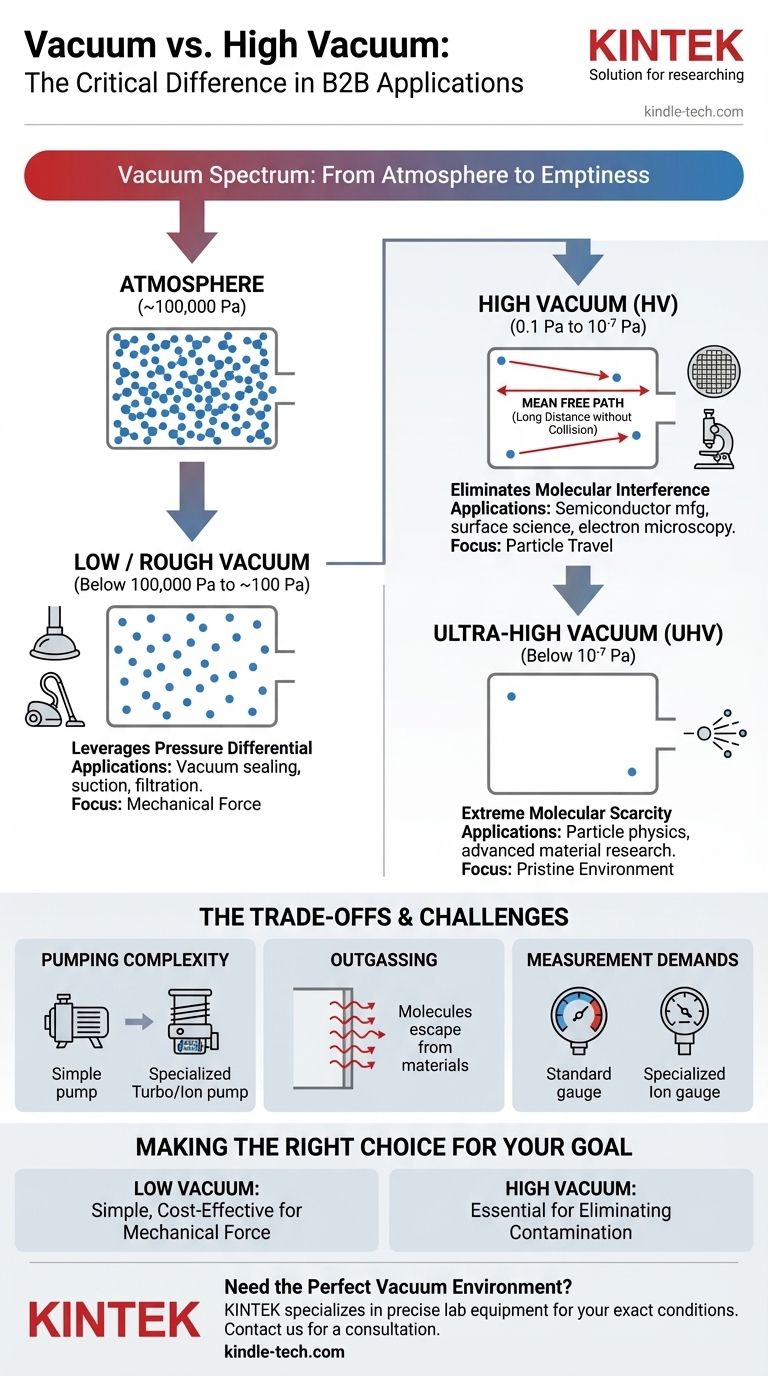
In technical terms, a "vacuum" is any space with a pressure lower than the surrounding atmospheric pressure. A "high vacuum," however, refers to a specific and much more rigorous standard of vacuum, typically in the pressure range of 0.1 to 10⁻⁷ Pascals, where the density of gas molecules is drastically reduced to a point where they rarely interact.
The crucial difference is not merely the pressure value, but the physical environment it creates. A general vacuum reduces air resistance, while a high vacuum creates an environment so devoid of molecules that their influence on a process becomes negligible.

From "Empty Space" to a Spectrum of Quality
The term "vacuum" is not a single state but a continuous spectrum. Understanding where "high vacuum" sits on this spectrum is key to grasping its importance.
Defining a General Vacuum
Any enclosed space with fewer gas molecules than the outside air is technically a vacuum. This is often called a rough or low vacuum.
Atmospheric pressure at sea level is approximately 100,000 Pascals (Pa). A low vacuum simply lowers this pressure, but still leaves trillions of molecules per cubic centimeter.
The Role of Molecular Density
The quality of a vacuum is fundamentally about the scarcity of molecules. Pressure is simply our most convenient way to measure this density.
As you pump gas out of a chamber, the pressure drops because the number of molecules per unit of volume decreases. A better vacuum is simply a more empty space.
What Makes a Vacuum "High"
A high vacuum is achieved when the pressure is reduced so dramatically that the behavior of the remaining molecules changes.
In this state, the mean free path—the average distance a molecule travels before colliding with another—can become longer than the chamber itself. Particles can now travel from one end to the other without interference, a critical property for many advanced applications.
Why This Distinction Is Critical
The difference between a low and high vacuum dictates what is physically possible within that environment. Different tasks require fundamentally different levels of "emptiness."
Low Vacuum Applications
Low vacuums are used when the primary goal is to leverage a pressure differential.
Examples include vacuum cleaners, food sealing, or suction cups. These applications only require removing enough air to create a force; the remaining molecules are irrelevant.
High and Ultra-High Vacuum Applications
High vacuum is essential when the goal is to eliminate molecular interference.
This is non-negotiable in fields like semiconductor manufacturing, particle accelerators, and mass spectrometry, where stray molecules would collide with electron beams or contaminate sensitive surfaces, rendering the process useless.
Understanding the Trade-offs
Moving from a low to a high vacuum introduces significant complexity and cost. It is not a trivial step.
The Challenge of Pumping
Achieving a high vacuum requires multiple stages of specialized pumps. A simple mechanical pump can create a low vacuum, but a turbomolecular or ion pump is needed to capture the few remaining molecules to reach high vacuum levels.
The Problem of Outgassing
At very low pressures, the primary source of gas is no longer the air you're pumping out, but the materials of the chamber itself. Molecules trapped within the metal walls begin to escape in a process called outgassing, which actively works against achieving a higher vacuum.
The Demands of Measurement
Standard pressure gauges do not work at high vacuum levels. Specialized instruments, such as ion gauges, are required to measure the tiny number of remaining molecules, adding to the system's complexity.
Making the Right Choice for Your Goal
Selecting the correct vacuum level is a critical engineering decision driven entirely by the needs of your process.
- If your primary focus is creating mechanical force or removing bulk air: A low or rough vacuum is almost always sufficient, simpler, and more cost-effective.
- If your primary focus is preventing atomic-level contamination or ensuring particles can travel unimpeded: A high or ultra-high vacuum is an absolute requirement.
Ultimately, understanding this spectrum allows you to engineer the precise environment your objective demands, ensuring success without unnecessary expense or complexity.
Summary Table:
| Vacuum Type | Typical Pressure Range | Primary Applications | Key Characteristic |
|---|---|---|---|
| Low / Rough Vacuum | Below 100,000 Pa to ~100 Pa | Vacuum sealing, suction, filtration | Creates a pressure differential for mechanical force |
| High Vacuum (HV) | 0.1 Pa to 10⁻⁷ Pa | Semiconductor manufacturing, surface science, electron microscopy | Eliminates molecular interference; long mean free path |
| Ultra-High Vacuum (UHV) | Below 10⁻⁷ Pa | Particle physics, advanced material research | Extreme molecular scarcity; minimal outgassing environment |
Need to Create the Perfect Vacuum Environment for Your Application?
Choosing the right vacuum level is critical for the success of your laboratory processes, from basic filtration to advanced thin-film deposition. KINTEK specializes in providing the precise lab equipment and consumables you need to achieve and maintain the exact vacuum conditions your work demands.
We help you:
- Select the right pumps and gauges for your target pressure range.
- Understand the challenges of outgassing and system design.
- Optimize your setup for efficiency and reliability, avoiding unnecessary complexity.
Let's discuss your specific vacuum requirements. Our experts are ready to help you build a solution that ensures precision and repeatability in your lab.
Contact KINTEK today for a consultation and let us be your partner in laboratory excellence.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Laboratory Vertical Water Circulating Vacuum Pump for Lab Use
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Laboratory Rotary Vane Vacuum Pump for Lab Use
People Also Ask
- What is sintering and melting in additive manufacturing? Choose the Right Process for Your Part's Strength and Density
- What are the advantages of powder sintering? Unlock Superior Strength, Conductivity & Translucency
- How does a vacuum furnace aid titanium diffusion bonding? Unlock High-Performance Multilayer Laminate Manufacturing
- What is the necessity of using a vacuum drying oven for nZVI catalysts? Protect Reactivity and Prevent Oxidation
- What changes in the annealing process? A Guide to the 3 Key Microstructural Stages
- What are the disadvantages of hardening process? Understanding the Trade-offs of Increased Strength
- What are three types of carburizing? A Guide to Pack, Gas, and Liquid Methods
- How do you braze a furnace? A Guide to High-Volume, Precision Metal Joining



















