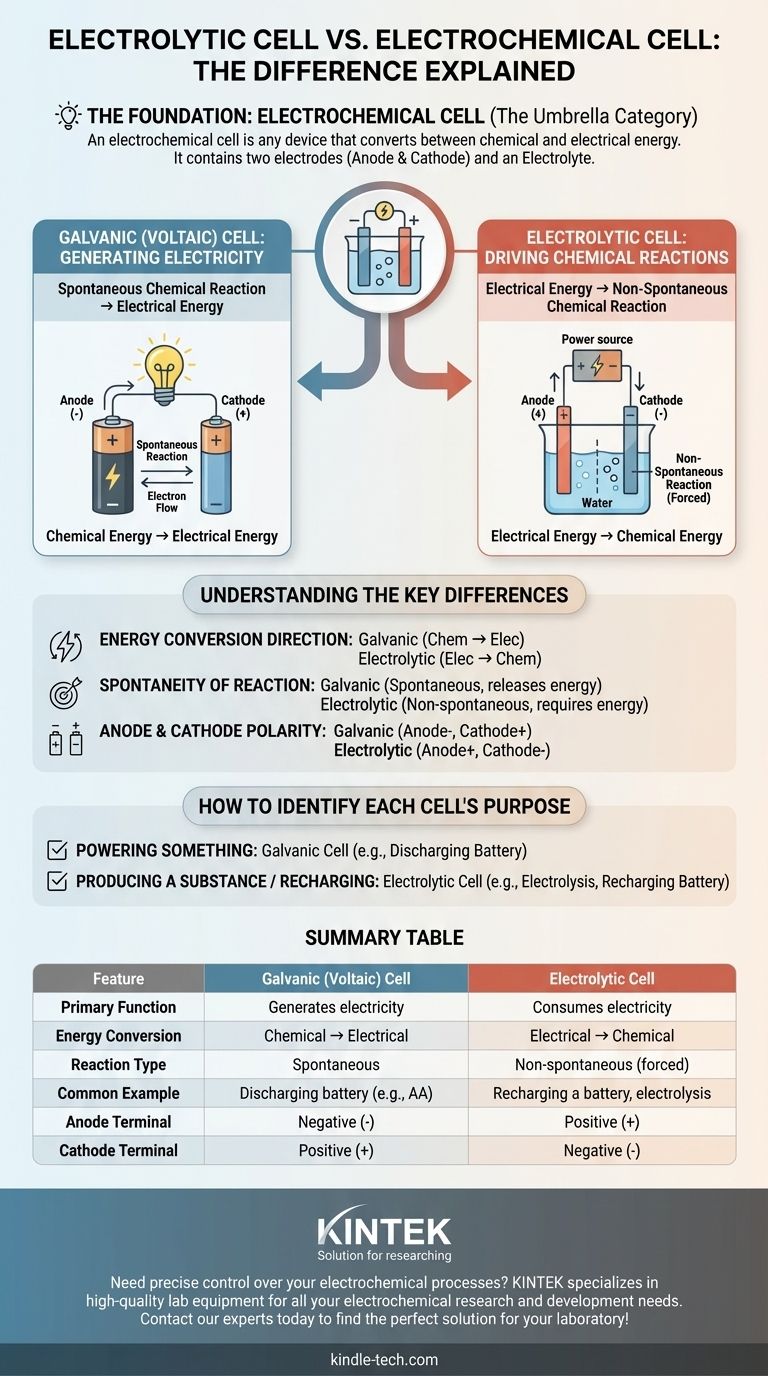
The primary source of confusion is that an electrolytic cell is not a separate concept from an electrochemical cell—it is one of the two main types of electrochemical cells. An electrochemical cell is the broad category for any device that converts between chemical and electrical energy. The real distinction lies between galvanic (voltaic) cells, which generate electricity from chemical reactions, and electrolytic cells, which use electricity to cause chemical reactions.
The term electrochemical cell is the umbrella category. The two specific types under this umbrella are galvanic cells (which create electricity) and electrolytic cells (which consume electricity). Their fundamental difference is the direction of energy conversion.

The Foundation: What Is an Electrochemical Cell?
Core Components
An electrochemical cell is any device containing two electrodes (an anode and a cathode) and an electrolyte. These components work together to facilitate the transfer of electrons, either creating or consuming an electric current.
The Overarching Purpose
The sole purpose of an electrochemical cell is to serve as a bridge between chemical energy and electrical energy. The direction of this energy conversion determines the specific type of cell.
The Two Fundamental Types of Conversion
Galvanic (Voltaic) Cells: Generating Electricity
A galvanic cell, also known as a voltaic cell, harnesses a spontaneous chemical reaction to produce electrical energy.
Think of a standard AA battery. The chemical materials inside it react naturally, releasing energy in the form of an electric current that can power a device. This is a galvanic cell in action.
Electrolytic Cells: Driving Chemical Reactions
An electrolytic cell does the exact opposite. It uses an external source of electrical energy (like a power supply) to force a non-spontaneous chemical reaction to occur.
A common example is electrolysis, such as splitting water into hydrogen and oxygen. This reaction does not happen on its own; it requires a constant input of electricity, which is the function of the electrolytic cell. Recharging a battery is another example of this process.
Understanding the Key Differences
Energy Conversion Direction
This is the most critical distinction. A galvanic cell converts stored chemical energy into electrical energy. An electrolytic cell converts supplied electrical energy into chemical energy.
Spontaneity of the Reaction
The chemical reaction in a galvanic cell is spontaneous—it proceeds naturally and releases energy.
The reaction in an electrolytic cell is non-spontaneous—it requires a constant input of external energy to proceed.
Anode and Cathode Polarity
This is a common point of confusion but follows logically from the energy flow.
In a galvanic cell (discharging battery), the anode is the source of electrons, making it the negative terminal, while the cathode is the positive terminal.
In an electrolytic cell (recharging battery), an external power source reverses the flow. The anode is where oxidation still occurs but is forced to be the positive terminal, and the cathode is the negative terminal.
How to Identify Each Cell's Purpose
Use the cell's primary function as your guide to easily distinguish between the two types.
- If the primary goal is to power something: You are using a galvanic (voltaic) cell, as a spontaneous chemical reaction is producing electricity.
- If the primary goal is to produce a substance or recharge a battery: You are using an electrolytic cell, as electricity is being consumed to force a chemical reaction.
Ultimately, both are simply two sides of the same electrochemical coin, defined by whether they generate or consume electrical power.
Summary Table:
| Feature | Galvanic (Voltaic) Cell | Electrolytic Cell |
|---|---|---|
| Primary Function | Generates electricity | Consumes electricity |
| Energy Conversion | Chemical → Electrical | Electrical → Chemical |
| Reaction Type | Spontaneous | Non-spontaneous (forced) |
| Common Example | Discharging battery (e.g., AA) | Recharging a battery, electrolysis |
| Anode Terminal | Negative (-) | Positive (+) |
| Cathode Terminal | Positive (+) | Negative (-) |
Need precise control over your electrochemical processes? KINTEK specializes in high-quality lab equipment for all your electrochemical research and development needs. Whether you are developing new battery technologies or synthesizing materials via electrolysis, our reliable instruments ensure accurate and reproducible results. Contact our experts today to find the perfect solution for your laboratory!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What materials are used for the caps of the sealed and non-sealed electrolysis cells? PTFE vs. POM Explained
- How does a Solid Oxide Electrolyzer Cell (SOEC) achieve higher energy efficiency? Unlock Superior Hydrogen Production
- What types of electrodes are used in the electrolytic cell? Choose the Right System for Your Lab
- What safety precautions and operational guidelines should be followed with H-type electrolytic cells for safe lab use?
- Why are an electrolytic polishing system and specific electrolytes necessary for Inconel 625? Expert Analysis
- How does a specialized electrolytic cell achieve concentration? Boost Tritium Detection with Isotope Enrichment
- What precaution should be taken regarding voltage control during an experiment? Avoid System Failure with Proper Voltage Limits
- What is the purpose of using oxalic acid solution for electrolytic polishing? Enhance Metallographic Clarity



















