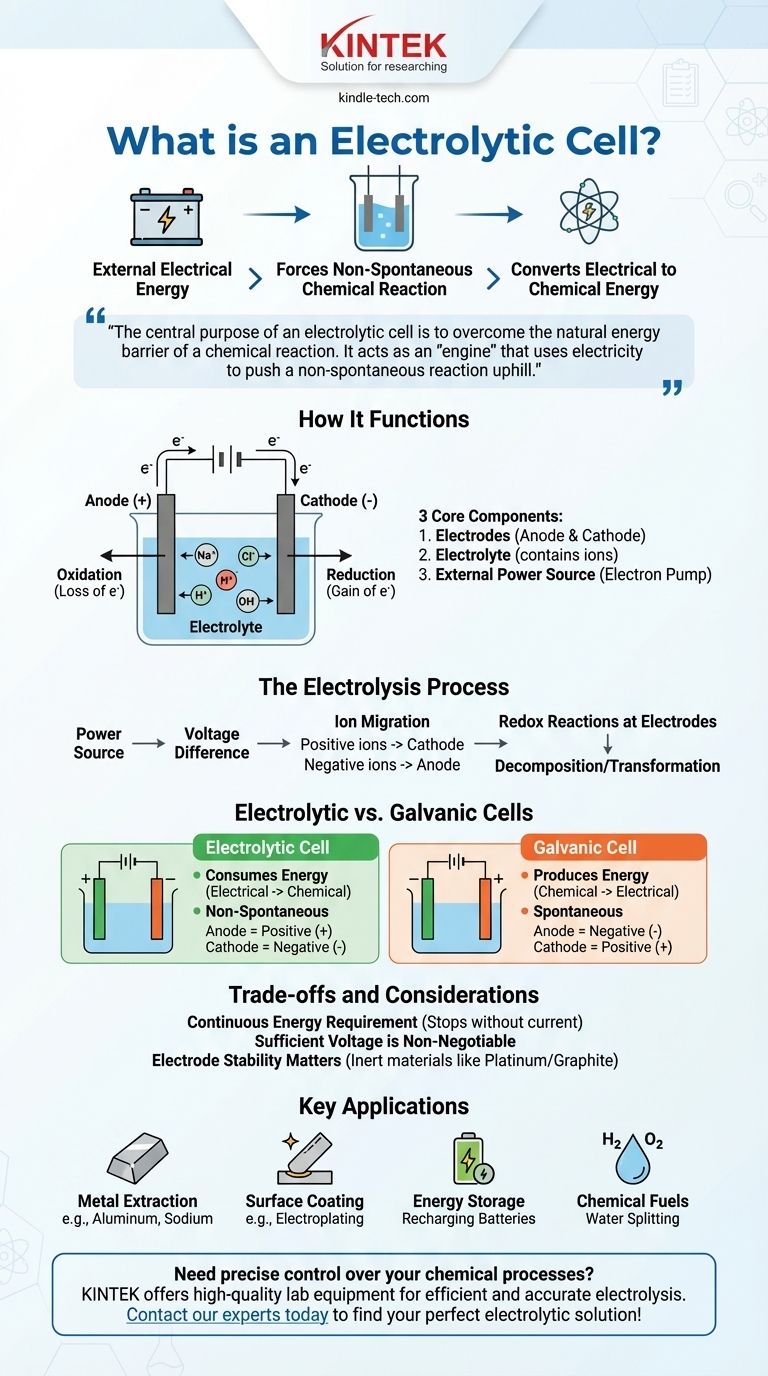
In essence, an electrolytic cell is a device that uses electrical energy from an external source, like a battery, to force a chemical reaction that would not happen on its own. This process, known as electrolysis, converts electrical energy into stored chemical energy by decomposing compounds like water or molten salts.
The central purpose of an electrolytic cell is to overcome the natural energy barrier of a chemical reaction. It acts as an "engine" that uses electricity to push a non-spontaneous reaction uphill, accomplishing tasks like separating elements or charging a battery.

How an Electrolytic Cell Functions
To understand an electrolytic cell, we must look at its essential parts and the process they enable. The entire system is designed to control the flow of electrons and ions to drive a specific chemical change.
The Three Core Components
An electrolytic cell is built from three primary parts.
-
Electrodes (Anode and Cathode): These are two metallic or electronic conductors that serve as the sites for the chemical reaction. The anode is the positive electrode where oxidation (loss of electrons) occurs, and the cathode is the negative electrode where reduction (gain of electrons) occurs.
-
Electrolyte: This is a substance, typically a liquid solution or a molten salt, that contains free-moving ions. The electrolyte allows charge to flow between the two electrodes, completing the electrical circuit.
-
External Power Source: This is a crucial component, like a battery or DC power supply. It acts as an "electron pump," pulling electrons away from the anode and pushing them onto the cathode.
The Process of Electrolysis
The power source creates a voltage difference across the electrodes.
This forces ions in the electrolyte to migrate. Positively charged ions move toward the negative cathode, and negatively charged ions move toward the apositive anode.
At the electrodes, redox (charge-transfer) reactions occur. At the anode, substances lose electrons, and at the cathode, substances gain electrons, resulting in the decomposition or transformation of the electrolyte's components.
The Critical Distinction: Electrolytic vs. Galvanic Cells
A common point of confusion is the difference between an electrolytic cell and its counterpart, the galvanic (or voltaic) cell, which is what we typically think of as a standard battery.
Energy Conversion
An electrolytic cell consumes energy. It converts electrical energy into chemical energy.
A galvanic cell produces energy. It converts stored chemical energy into electrical energy.
Reaction Spontaneity
The reaction in an electrolytic cell is non-spontaneous. It requires an external energy input to proceed.
The reaction in a galvanic cell is spontaneous. It occurs naturally, releasing energy in the process.
Electrode Polarity
In an electrolytic cell, the anode is positive and the cathode is negative. This is because the external power source dictates the charge.
In a galvanic cell, the anode is negative and the cathode is positive. The spontaneous chemical reaction itself determines the charge.
Understanding the Trade-offs and Considerations
While powerful, electrolytic cells operate under specific constraints that are important to recognize.
A Continuous Energy Requirement
The primary characteristic of an electrolytic cell is its dependence on an external power source. The process of electrolysis stops the moment the electrical current is removed.
Sufficient Voltage is Non-Negotiable
The external voltage applied must be high enough to overcome the natural resistance of the non-spontaneous reaction. If the voltage is too low, no chemical change will occur.
Electrode Stability Matters
The electrodes themselves must be chosen carefully. In many processes, they are made of inert materials like platinum or graphite that facilitate the reaction without being consumed. In other applications, the electrode material is intentionally chosen to participate in the reaction, as seen in some types of purification.
Key Applications of Electrolytic Cells
The ability to force chemical reactions gives electrolytic cells a central role in industry and technology.
- If your primary focus is producing pure elements: Electrolysis is used to extract metals like aluminum from its ore (bauxite) or to produce sodium and chlorine gas from molten sodium chloride.
- If your primary focus is surface coating: The process of electroplating uses an electrolytic cell to deposit a thin layer of one metal (like chromium or gold) onto another for protection or decoration.
- If your primary focus is energy storage: Recharging a battery involves running it as an electrolytic cell. The external charger forces ions back to their original state, storing energy for later use.
- If your primary focus is creating chemical fuels: Electrolytic cells can split water (H₂O) into hydrogen gas and oxygen gas, with the hydrogen serving as a clean fuel source.
By applying electrical energy with precision, an electrolytic cell gives us direct control over chemical transformations.
Summary Table:
| Feature | Electrolytic Cell | Galvanic Cell (Battery) |
|---|---|---|
| Energy Conversion | Converts electrical energy to chemical energy | Converts chemical energy to electrical energy |
| Reaction Type | Non-spontaneous (requires external power) | Spontaneous (occurs naturally) |
| Anode Charge | Positive | Negative |
| Primary Function | Driving desired chemical reactions (e.g., electroplating) | Generating electricity |
Need precise control over your chemical processes? KINTEK's high-quality lab equipment, including reliable power supplies and durable electrodes, is essential for efficient and accurate electrolysis. Whether your application is electroplating, metal purification, or energy storage research, our consumables and instruments are designed for superior performance. Contact our experts today to find the perfect electrolytic solution for your laboratory's needs!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- Why is an electrolytic cell system essential for evaluating the PEC performance of BiFeO3? Boost Research Precision
- Why are cooling systems essential for industrial-scale electrolysis cells? Manage Waste Heat for Peak Performance
- What is the process of electrolysis in an electrolytic cell? A Step-by-Step Guide to Driving Non-Spontaneous Reactions
- What are the requirements for an electrolytic cell with a quartz window? Ensure Accurate PEC Performance Tests
- Why are laboratory electrolytic polishing and etching systems necessary? Reveal the Microstructure of Stainless Steel
- What role does an electrolytic cell system play in the fabrication of TiO2 nanotube arrays? Control Your Nanostructure
- What types of electrodes are used in an H-type electrolytic cell? A Guide to the Essential Three-Electrode System
- How does a laboratory electrochemical anodization setup achieve the controlled growth of titanium dioxide nanotubes?



















