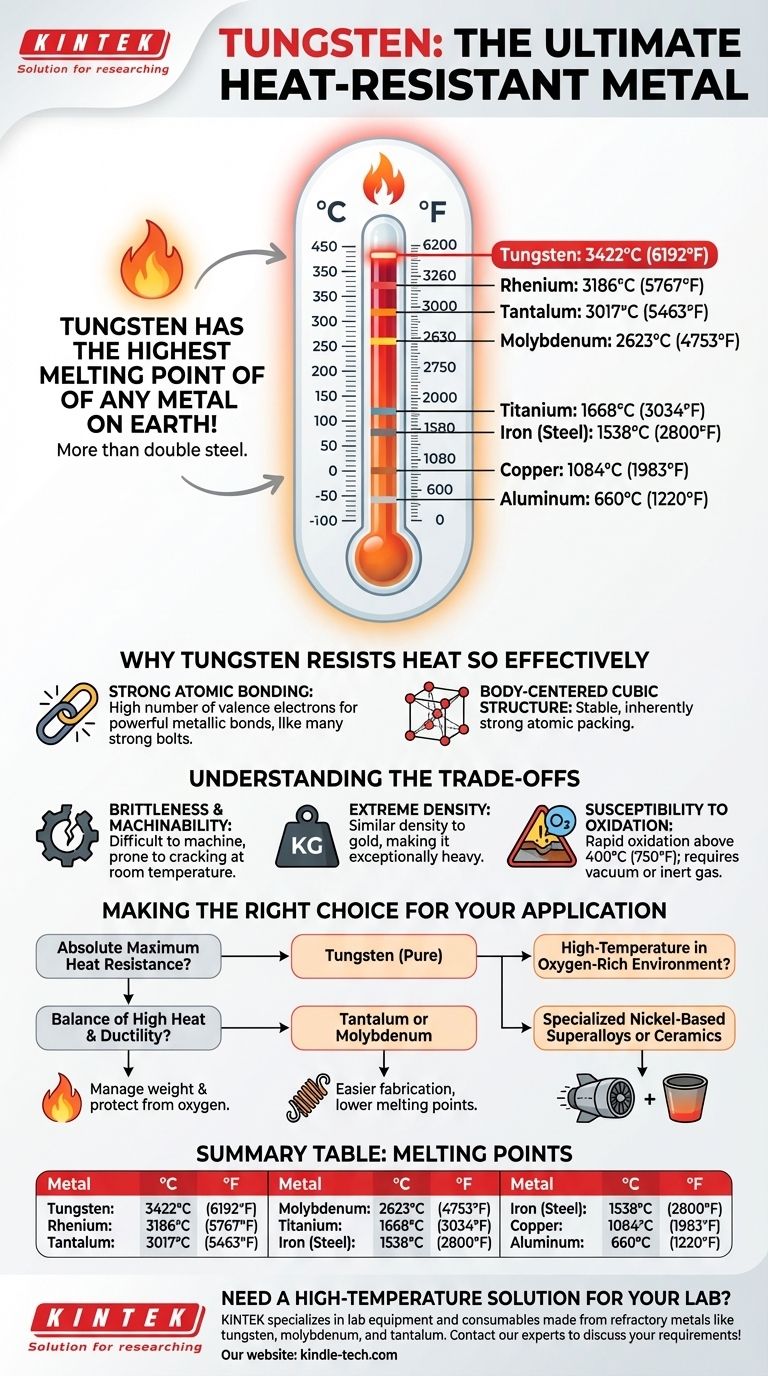
To be direct, tungsten has the highest melting point of any metal on Earth. At 3422°C (6192°F), it melts at a temperature more than double that of steel. This singular property makes it foundational for applications where other metals would simply turn to liquid.
The critical insight is not just that tungsten has a high melting point, but why. Its uniquely strong atomic structure makes it the ultimate material for extreme heat applications, but this same structure introduces significant trade-offs in density and workability that must be carefully managed.

A Visual Comparison: Tungsten vs. Other Metals
To understand the scale of tungsten's heat resistance, it's best to see it in context. Its peers in the "refractory metals" group are the only ones that come close, while common industrial metals are left far behind.
| Metal | Category | Melting Point (°C) | Melting Point (°F) |
|---|---|---|---|
| Tungsten | Refractory | 3422°C | 6192°F |
| Rhenium | Refractory | 3186°C | 5767°F |
| Tantalum | Refractory | 3017°C | 5463°F |
| Molybdenum | Refractory | 2623°C | 4753°F |
| Titanium | Common Industrial | 1668°C | 3034°F |
| Iron (Steel Base) | Common Industrial | 1538°C | 2800°F |
| Copper | Common Industrial | 1084°C | 1983°F |
| Aluminum | Common Industrial | 660°C | 1220°F |
Why Tungsten Resists Heat So Effectively
Tungsten's performance isn't magic; it's a result of its fundamental atomic properties. Two factors are primarily responsible for its incredible stability.
The Power of Atomic Bonding
Tungsten has a very high number of valence electrons—the outer electrons that form bonds between atoms. This creates an extremely dense and powerful metallic bond.
Think of it as a structure held together with an exceptional number of incredibly strong bolts. A tremendous amount of thermal energy (heat) is required to vibrate the atoms enough to break these bonds and allow the material to melt.
The Body-Centered Cubic Structure
These powerfully bonded atoms are packed into a stable crystal lattice known as a Body-Centered Cubic (BCC) structure. This configuration is inherently strong and contributes to the material's overall stability at high temperatures.
Understanding the Trade-offs
A material with such extreme properties rarely comes without significant drawbacks. For all its strength, tungsten presents serious engineering challenges.
Brittleness and Machinability
At room temperature, tungsten is notoriously brittle. This makes it very difficult to machine, form, or work with compared to materials like steel or aluminum. It tends to crack or shatter under stress rather than bend.
Extreme Density
Tungsten is one of the densest metals, with a density similar to that of gold. This makes it exceptionally heavy, precluding its use in applications where weight is a primary concern, such as most general aerospace structures.
Susceptibility to Oxidation
While it can withstand incredible heat, tungsten cannot do so in the presence of oxygen. It begins to oxidize rapidly at temperatures above 400°C (750°F). Therefore, it must be used in a vacuum or protected by an inert gas atmosphere for high-temperature applications.
Making the Right Choice for Your Application
Selecting the right material requires balancing its primary benefit against its inherent trade-offs.
- If your primary focus is absolute maximum heat resistance: Tungsten is your only choice among pure metals, provided you can manage its weight and protect it from oxygen.
- If you need a balance of high heat resistance and better ductility: Consider its refractory peers like Tantalum or Molybdenum, which are easier to fabricate but have lower melting points.
- If you require high-temperature performance in an oxygen-rich environment: You must look beyond pure refractory metals to specialized nickel-based superalloys or ceramics.
Understanding these properties allows you to select a material based not just on a single data point, but on its true performance profile for the task at hand.
Summary Table:
| Metal | Melting Point (°C) | Melting Point (°F) |
|---|---|---|
| Tungsten | 3422°C | 6192°F |
| Rhenium | 3186°C | 5767°F |
| Tantalum | 3017°C | 5463°F |
| Molybdenum | 2623°C | 4753°F |
| Titanium | 1668°C | 3034°F |
| Iron (Steel) | 1538°C | 2800°F |
Need a high-temperature solution for your lab? KINTEK specializes in lab equipment and consumables made from refractory metals like tungsten, molybdenum, and tantalum. Our expertise ensures you get the right material for extreme heat applications, balancing performance with manufacturability. Contact our experts today to discuss your specific high-temperature requirements!
Visual Guide
Related Products
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Custom PTFE Teflon Parts Manufacturer for Reagent Wide Mouth Fine Mouth Sample High Temperature Bottles
- High Temperature Wear-Resistant Alumina Al2O3 Plate for Engineering Advanced Fine Ceramics
- Precision Machined Silicon Nitride (SiN) Ceramic Sheet for Engineering Advanced Fine Ceramics
People Also Ask
- Can tungsten be used as a heating element? Unlocking Extreme Heat for High-Temperature Applications
- What is the function of high-temperature metal filaments in HFCVD? Catalyzing Diamond Growth Success
- What is the suitability of tungsten as an electrical conducting material for heating applications? Master Extreme High-Temperature Heating
- What happens when tungsten is heated? Harnessing Extreme Heat for Demanding Applications
- What is the melting point of tungsten? Discover the Metal That Withstands Extreme Heat



















