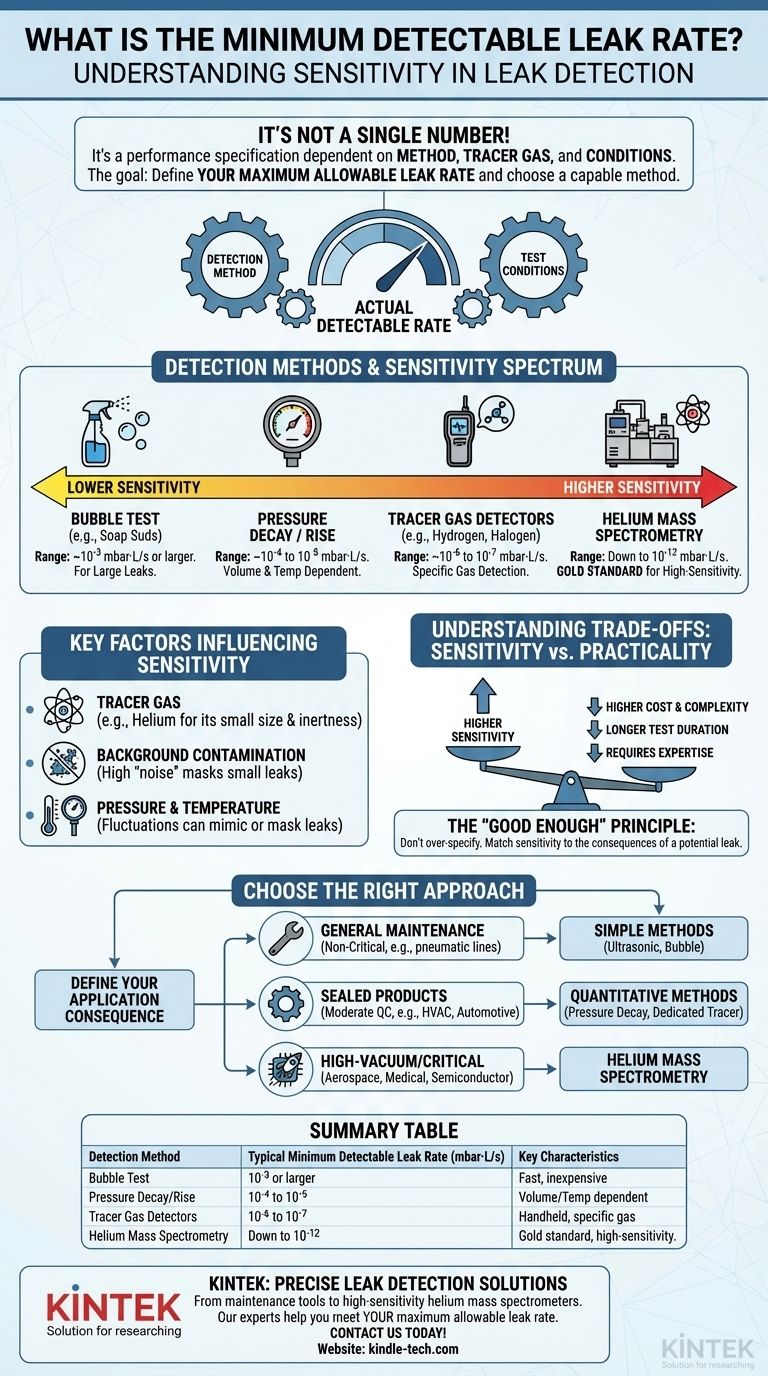
The minimum detectable leak rate is not a single, universal number but rather a performance specification that is entirely dependent on the detection method, the specific tracer gas used, and the conditions of the test. While some methods can only find relatively large leaks, the most sensitive technology, helium mass spectrometry, can theoretically detect leak rates as low as 5x10⁻¹³ mbar·L/s under ideal laboratory conditions.
The core challenge is not finding the absolute smallest leak possible, but rather defining the maximum allowable leak rate for your specific application. You then select a detection method capable of reliably and efficiently finding leaks smaller than that critical threshold.

What Determines the Detectable Leak Rate?
The sensitivity of any leak test is governed by a few key variables. Understanding these variables is essential to choosing the right method for your goal, whether it's ensuring the integrity of a vacuum chamber or finding a costly air leak in a factory.
The Detection Method
Each method operates on a different principle and thus has a different inherent sensitivity.
- Bubble Test (e.g., Soap Suds): This is the simplest method. A solution is applied to a pressurized part, and escaping gas forms visible bubbles. It is fast and inexpensive but is only effective for larger leaks, typically in the range of 10⁻³ mbar·L/s or larger.
- Pressure Decay / Rise: This technique involves pressurizing (or evacuating) a sealed component and monitoring its internal pressure over time. A change in pressure indicates a leak. Its sensitivity is commonly around 10⁻⁴ to 10⁻⁵ mbar·L/s but is highly dependent on the test object's volume and temperature stability.
- Tracer Gas Detectors (e.g., Hydrogen, Halogen): These handheld electronic "sniffers" are tuned to detect a specific gas that has been introduced into the system. They are more sensitive than pressure decay, with typical minimum detectable leak rates of 10⁻⁶ to 10⁻⁷ mbar·L/s.
- Helium Mass Spectrometry: This is the gold standard for high-sensitivity leak detection. A mass spectrometer is tuned to detect only helium atoms, which are used as the tracer gas. Because helium is rare in the atmosphere and its atoms are very small, this method can reliably find exceptionally small leaks, often down to 10⁻¹² mbar·L/s.
Key Factors That Influence Sensitivity
Beyond the chosen method, several environmental and setup factors can dramatically impact the actual detectable leak rate in a real-world scenario.
The Choice of Tracer Gas
The physical properties of the tracer gas are critical. Helium is the preferred choice for high-sensitivity tests because it is inert, non-flammable, has a very small atomic size (allowing it to pass through tiny leak paths), and has a very low natural concentration in the atmosphere (about 5 ppm). Hydrogen is also used due to its small molecular size, but its flammability poses a safety risk.
Background Contamination
A detector can only identify a leak if it can distinguish the tracer gas signal from the "noise" of the surrounding environment. If the test area is contaminated with the tracer gas—for instance, from a previous test or a large, unrepaired leak—the background signal will be high. This makes it impossible to pinpoint a small leak, effectively increasing your minimum detectable rate.
Pressure and Temperature
Leak rates are defined as a volume of gas at a certain pressure moving per unit of time (e.g., mbar·L/s). Increasing the pressure difference across a leak path will increase the flow of gas, making the leak larger and easier to detect. Conversely, temperature fluctuations during a pressure decay test can cause the gas inside to expand or contract, mimicking a leak or mask and creating a false result.
Understanding the Trade-offs: Sensitivity vs. Practicality
Achieving the lowest possible leak rate detection comes at a cost. Selecting the right method involves balancing sensitivity with practical constraints.
Cost and Complexity
A bottle of soap solution is inexpensive, while a helium mass spectrometer leak detector is a significant capital investment that requires a skilled operator. The cost of the equipment and the required expertise generally scale directly with the sensitivity of the method.
Test Duration
High-sensitivity methods can be time-consuming. A pressure decay test on a large vessel may require hours for the pressure to stabilize and provide a measurable result. Meticulously "sniffing" every seam and joint on a complex piece of equipment with a helium leak detector also takes considerable time.
The "Good Enough" Principle
The objective is not always to achieve the lowest possible detection limit. A leak that is catastrophically large for a semiconductor vacuum chamber may be completely irrelevant in a compressed air system for pneumatic tools. Over-specifying your leak testing requirements leads to unnecessary expense and time.
How to Determine the Right Approach for Your Application
Base your decision on the consequences of a potential leak in your system.
- If your primary focus is general maintenance on non-critical systems (e.g., factory pneumatic lines): Start with the simplest methods like ultrasonic detectors or bubble solutions, as they are fast, cheap, and sufficient for finding financially significant leaks.
- If you are manufacturing sealed products with moderate quality standards (e.g., HVAC units, automotive components): You need a quantitative method like pressure decay or a dedicated tracer gas (e.g., hydrogen/nitrogen mix) to ensure you meet specific quality control specifications.
- If you are working with high-vacuum, high-purity, or safety-critical systems (e.g., aerospace, medical devices, semiconductor tools): Helium mass spectrometry is often the only acceptable choice, as the maximum allowable leak rates are far below what other methods can reliably detect.
Ultimately, effective leak detection is about matching the sensitivity of your method to the strictness of your requirements.
Summary Table:
| Detection Method | Typical Minimum Detectable Leak Rate (mbar·L/s) | Key Characteristics |
|---|---|---|
| Bubble Test | 10⁻³ or larger | Fast, inexpensive, for large leaks |
| Pressure Decay / Rise | 10⁻⁴ to 10⁻⁵ | Volume and temperature dependent |
| Tracer Gas Detectors | 10⁻⁶ to 10⁻⁷ | Handheld, specific gas detection |
| Helium Mass Spectrometry | Down to 10⁻¹² | Gold standard for high-sensitivity |
Need to find the right leak detection method for your lab or production line? KINTEK specializes in providing precise lab equipment and consumables for all your leak testing needs, from basic maintenance tools to high-sensitivity helium mass spectrometers. Our experts can help you select the ideal solution to meet your application's maximum allowable leak rate, ensuring efficiency, safety, and compliance. Contact us today to discuss your requirements and enhance your quality control process!
Visual Guide
Related Products
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
- KF/ISO/CF Ultra-High Vacuum Stainless Steel Flange Pipe/Straight Pipe/Tee/Cross
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What is the cost of a vacuum annealing furnace? Find the Right Price for Your Lab or Production Needs
- What materials are used in vacuum casting? A Guide to Silicone Molds and Polyurethane Resins
- How is the sintering temperature related to the melting temperature? A Guide to Solid-State Bonding
- Does heat treating change density? Yes, and Here’s Why It Matters for Precision
- What role do electric vacuum laboratory furnaces play in LBE corrosion tests? Ensure Precision Reactor Simulations
- What is the relationship between temperature and pressure in a vacuum? Mastering Thermal Control for Optimal Vacuum Performance
- What are the different design schemes and common mediums used for gas cooling in vacuum furnaces? Optimize Your Heat Treatment Process
- What is the effect of vacuum on heat transfer? Mastering Thermal Control in Extreme Environments













