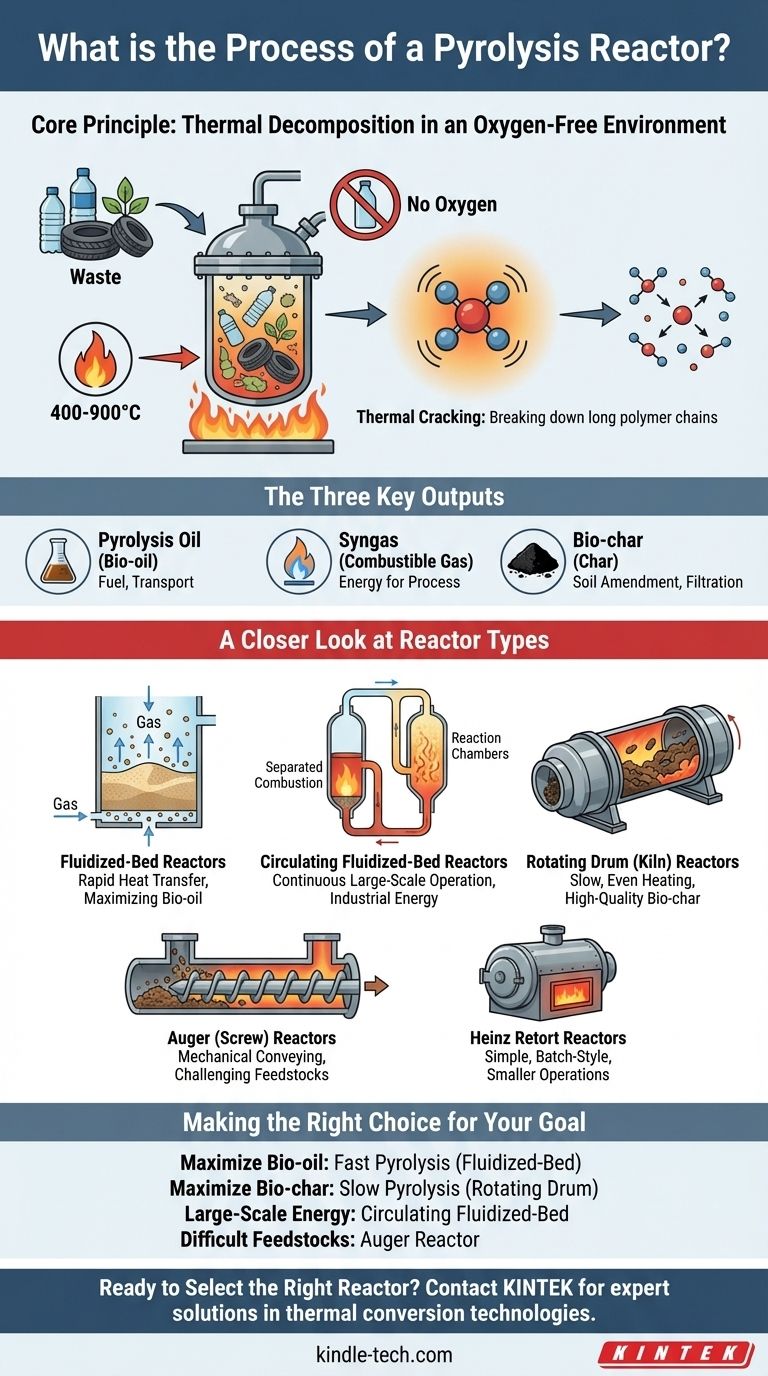
At its core, the pyrolysis reactor process is a form of thermal decomposition. It works by feeding waste material—such as plastics, tires, or biomass—into a sealed reactor and heating it to high temperatures (typically 400-900°C) in an environment with little to no oxygen. This intense heat, without the presence of oxygen to allow burning, causes the large, complex molecules in the material to break down into smaller, more valuable components: a liquid oil, a combustible gas, and a solid carbon char.
Pyrolysis is not about incinerating waste; it's a precise chemical engineering process designed to reclaim the energy and raw materials locked within it. The key is understanding that the type of reactor you use directly dictates the efficiency of the process and the ratio of oil, gas, and char you produce.

The Fundamental Principle: Heat Without Oxygen
The entire pyrolysis process hinges on one critical factor: creating an oxygen-free (anaerobic) environment. Preventing combustion is what allows for the transformation of waste into new products instead of just turning it into ash and heat.
What Happens Inside the Reactor?
The process is a form of thermal cracking, similar to what happens in a petroleum refinery. As the feedstock is heated, the long polymer chains that make up plastics or biomass begin to vibrate and break apart.
These larger molecules decompose into smaller, more volatile compounds. The lighter compounds vaporize into a gas, while heavier ones condense into a liquid, leaving behind a solid, carbon-rich residue.
The Three Key Outputs
The process consistently yields three primary products, each with its own use:
- Pyrolysis Oil (Bio-oil): A dark, viscous liquid that can be refined and used as an industrial fuel or upgraded into transportation fuels.
- Syngas (Synthesis Gas): A mixture of combustible gases (primarily hydrogen and carbon monoxide). This gas is often recycled to provide the heat needed to run the pyrolysis reactor itself, making the process more self-sufficient.
- Bio-char (Char): A stable, solid material rich in carbon. It can be used as an agricultural soil amendment to improve fertility, as a filtration medium (activated carbon), or as a solid fuel.
Why the Absence of Oxygen is Critical
If oxygen were present, the material would simply burn (combust), releasing its energy as heat and producing carbon dioxide and ash. By removing oxygen, the process forces the chemical bonds to break down thermally, preserving the chemical energy in the resulting oil and gas.
A Closer Look at Reactor Types
The "pyrolysis reactor" is not a single design but a category of technologies. The specific design dramatically influences the heat transfer rate, processing time, and final product yields.
Fluidized-Bed Reactors: For Speed and Efficiency
These reactors contain a bed of inert material, such as sand, which is "fluidized" by injecting a hot, inert gas (like nitrogen) from below. The feedstock is introduced into this turbulent bed of hot sand, resulting in extremely rapid and efficient heat transfer. This design is ideal for maximizing the yield of bio-oils from particulate matter like woody biomass.
Circulating Fluidized-Bed Reactors: For Large-Scale Operations
This is an evolution of the fluidized-bed design, where the heated bed material and char are continuously circulated between the reactor and a separate heating chamber. This technology is built for high-throughput, continuous operation, making it a common choice for large-scale renewable energy and electricity generation plants.
Rotating Drum (Kiln) Reactors: For Simplicity and Bio-char
This reactor is essentially a large, rotating, cylindrical drum housed within a furnace. The feedstock tumbles inside as the drum slowly rotates, ensuring even heating. This design typically results in slower pyrolysis, which favors the production of bio-char over bio-oil due to the longer residence time of the solids.
Auger (Screw) Reactors: For Mechanical Control
An auger reactor uses a large, motor-driven screw to actively push feedstock through a heated tube. Heat transfer relies on the mechanical force and pressure of the material being conveyed against the hot surfaces. This robust, mechanical approach can be advantageous for processing less uniform or more challenging feedstocks.
Heinz Retort Reactors: The "Oven" Approach
This is one of the simplest designs, consisting of an airtight vessel that is heated externally, much like an oven. Heat is transferred slowly through the reactor walls to the material inside. This method is often used for smaller, batch-style processes where speed is not the primary concern.
Understanding the Trade-offs
Choosing a pyrolysis reactor involves balancing competing priorities. There is no single "best" type; the optimal choice depends entirely on the goal.
Speed vs. Product Yield
Fast pyrolysis, achieved in fluidized-bed reactors, maximizes the production of liquid bio-oil because vapors are removed quickly before they can break down further. Slow pyrolysis, common in drum reactors, allows more time for secondary reactions, which increases the yield of solid bio-char.
Feedstock Flexibility
Reactors that rely on fluid dynamics, like fluidized beds, work best with dry, uniformly sized particles. Mechanically driven systems like auger reactors can often handle a wider variety of feedstock sizes and moisture contents, though potentially at a cost to thermal efficiency.
Complexity vs. Efficiency
Fluidized-bed reactors offer superior heat transfer and efficiency but are more complex and demanding to operate and maintain. Simpler designs like a retort or drum kiln have lower capital costs and are easier to operate but typically have lower throughput and thermal efficiency.
Making the Right Choice for Your Goal
Your selection of a pyrolysis reactor must be driven by your feedstock and your desired primary output.
- If your primary focus is maximizing liquid fuel (bio-oil) production: A fast pyrolysis system like a fluidized-bed reactor is your best choice due to its rapid heat transfer.
- If your primary focus is producing high-quality bio-char for agriculture: A slow pyrolysis system, such as a rotating drum reactor, will provide the longer residence time needed.
- If you are running a large-scale, continuous energy generation plant: A circulating fluidized-bed reactor provides the necessary scale and thermal efficiency for industrial throughput.
- If you are processing difficult, sticky, or non-uniform feedstock: An auger reactor's robust mechanical handling may be more suitable than a fluid-dynamic system.
Understanding these core designs empowers you to select the right pyrolysis technology to transform a waste liability into a valuable resource.
Summary Table:
| Reactor Type | Key Characteristic | Ideal For |
|---|---|---|
| Fluidized-Bed | Rapid heat transfer via hot, inert gas | Maximizing bio-oil yield |
| Circulating Fluidized-Bed | Continuous circulation for large-scale operation | Industrial energy generation |
| Rotating Drum (Kiln) | Slow, tumbling motion for even heating | High-quality bio-char production |
| Auger (Screw) | Mechanical conveying for challenging feedstocks | Processing non-uniform materials |
| Heinz Retort | Simple, batch-style external heating | Smaller, simpler operations |
Ready to select the right pyrolysis reactor for your specific waste stream and output goals? The experts at KINTEK are here to help. We specialize in providing high-quality lab equipment and consumables for research and development in thermal conversion technologies. Whether you're scaling up from lab tests or optimizing an industrial process, our team can provide the equipment and support you need to efficiently transform waste plastics, tires, or biomass into valuable oil, gas, and char.
Contact KINTEL today to discuss your project requirements and discover the ideal pyrolysis solution for your laboratory or facility.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















