At their core, carbon nanomaterials are substances made primarily of carbon atoms, with at least one dimension sized between 1 and 100 nanometers. Their structure is not a single form, but rather a family of different arrangements, known as allotropes. The most fundamental structures are two-dimensional graphene sheets, one-dimensional carbon nanotubes, and zero-dimensional fullerenes, with each structure giving rise to a unique set of properties.
The specific atomic arrangement—whether a flat sheet, a rolled tube, or a closed sphere—is the single most important factor that dictates a carbon nanomaterial's unique electrical, mechanical, and thermal properties. Understanding this link between structure and function is the key to their application.

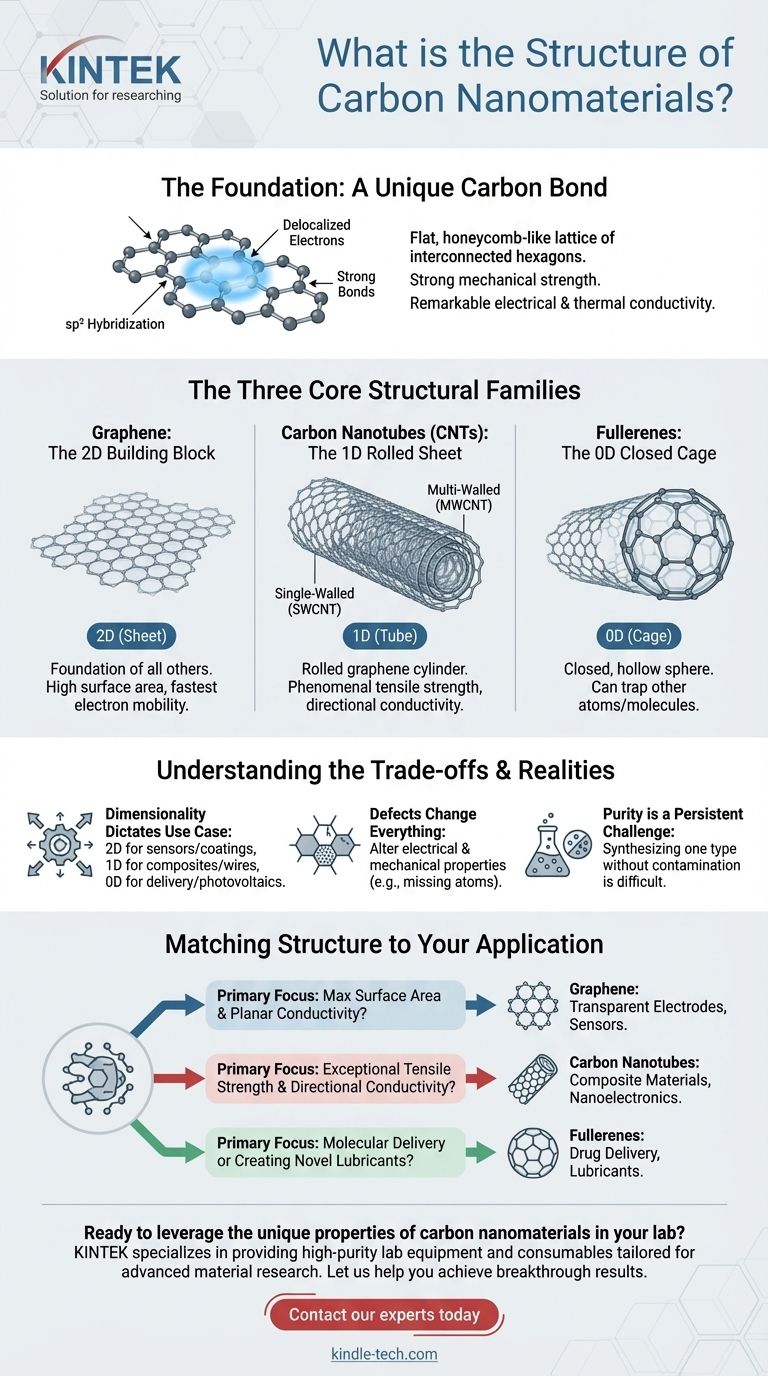
The Foundation: A Unique Carbon Bond
All major carbon nanomaterials are built from a special arrangement of carbon atoms. Understanding this is the first step to understanding their structure.
The Power of sp² Hybridization
Carbon atoms in these nanomaterials are typically joined by sp² hybridization. This type of bond creates a flat, honeycomb-like lattice of interconnected hexagons.
Imagine a floor tiled perfectly with hexagonal tiles; this is the basic two-dimensional pattern that carbon atoms form. This structure is incredibly stable and strong.
Why This Bond Matters
This hexagonal network is responsible for two key features. First, the bonds between the carbon atoms are among the strongest known, which gives materials like graphene immense mechanical strength.
Second, this structure leaves a sea of "delocalized" electrons that can move freely across the entire sheet. This is the source of their remarkable electrical and thermal conductivity.
The Three Core Structural Families
Based on this hexagonal building block, carbon nanomaterials are primarily classified by their dimensionality—how the sheet is arranged in space.
Graphene: The 2D Building Block
Graphene is the simplest carbon nanomaterial. It is a single, flat sheet of sp²-bonded carbon atoms, just one atom thick.
Think of it as the foundational material or the "mother" of all other graphitic carbons. Its two-dimensional structure provides an enormous surface area and the fastest known electron mobility at room temperature.
Carbon Nanotubes (CNTs): The 1D Rolled Sheet
Carbon Nanotubes (CNTs) are what you get if you take a sheet of graphene and roll it up into a seamless cylinder. This creates a one-dimensional structure.
CNTs can be single-walled (SWCNTs), consisting of a single rolled-up graphene cylinder, or multi-walled (MWCNTs), which are like concentric tubes nested inside one another. Their tubular structure gives them phenomenal tensile strength along their length.
Fullerenes: The 0D Closed Cage
Fullerenes are created when a graphene sheet is wrapped up to form a completely closed, hollow sphere or ellipsoid. This makes them zero-dimensional nanomaterials.
The most famous example is Buckminsterfullerene (C60), which has a soccer-ball shape made of 60 carbon atoms arranged in hexagons and pentagons. These cages can be used to trap other atoms or molecules inside.
Understanding the Trade-offs and Realities
The ideal structures described above are a starting point. In practice, several factors influence their real-world performance.
Dimensionality Dictates Use Case
The structure's dimension directly suggests its best use. Graphene's 2D plane is ideal for sensors, coatings, and membranes. A CNT's 1D tube is perfect for reinforcing composites or creating conductive wires. A fullerene's 0D cage is suited for drug delivery or as a component in photovoltaics.
Defects Change Everything
Real-world carbon nanomaterials are rarely perfect. Structural defects, such as missing atoms or the presence of pentagons in a graphene sheet, can dramatically alter electrical and mechanical properties. While sometimes undesirable, these defects can also be intentionally introduced to tune a material's behavior.
Purity is a Persistent Challenge
Synthesizing one type of nanomaterial without contamination from others (e.g., making pure CNTs without residual catalyst particles or amorphous carbon) is a significant challenge. The purity of the material is just as important as its ideal structure for achieving high performance.
Matching Structure to Your Application
Choosing the right nanomaterial requires aligning its inherent structure with your primary goal.
- If your primary focus is maximum surface area and planar conductivity: Graphene's flat, 2D sheet structure makes it the definitive choice for applications like transparent electrodes and sensors.
- If your primary focus is exceptional tensile strength and directional conductivity: Carbon nanotubes are the ideal choice, as their 1D tubular form provides unparalleled strength-to-weight and guides electrical flow along their axis.
- If your primary focus is molecular delivery or creating novel lubricants: Fullerenes offer a unique 0D cage structure that can encapsulate other molecules and act as nanoscale ball bearings.
Ultimately, understanding the atomic architecture of a carbon nanomaterial is the first step toward harnessing its revolutionary potential.
Summary Table:
| Structure | Dimensionality | Key Characteristics | Common Applications |
|---|---|---|---|
| Graphene | 2D (Sheet) | Single atom thick, high surface area, excellent planar conductivity | Sensors, transparent electrodes, coatings |
| Carbon Nanotubes (CNTs) | 1D (Tube) | High tensile strength, directional conductivity, can be single/multi-walled | Composite materials, nanoelectronics, conductive wires |
| Fullerenes (e.g., C60) | 0D (Cage) | Hollow spherical/ellipsoidal structure, can encapsulate molecules | Drug delivery, lubricants, photovoltaics |
Ready to leverage the unique properties of carbon nanomaterials in your lab?
At KINTEK, we specialize in providing high-purity lab equipment and consumables tailored for advanced material research. Whether you're working with graphene, CNTs, or fullerenes, our products ensure the precision and reliability your experiments demand.
Let us help you achieve breakthrough results. Contact our experts today to discuss how we can support your specific laboratory needs and drive your innovations forward.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- High-Purity Titanium Foil and Sheet for Industrial Applications
- High Purity Zinc Foil for Battery Lab Applications
People Also Ask
- What is the maximum working temperature of graphite? Unlock High-Temp Performance with the Right Atmosphere
- Can graphite withstand high-temperature? Maximizing Performance in Controlled Atmospheres
- Why can graphite withstand heat? Unlocking Its Extreme Thermal Stability for Your Lab
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- How much temperature can graphite withstand? Unlock its true potential up to 3000°C