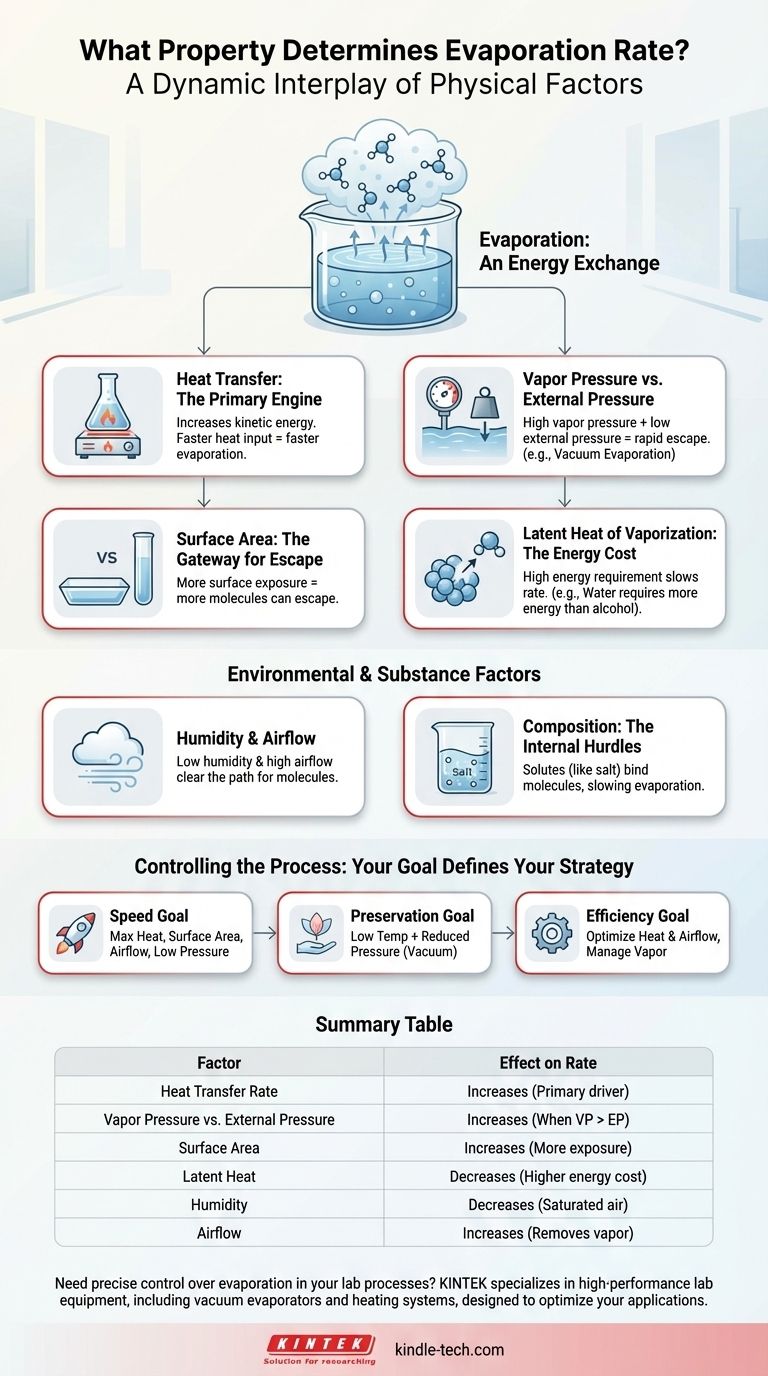
Evaporation is not governed by a single property, but rather by a dynamic interplay of several key physical factors. The rate is fundamentally determined by the speed of heat transfer into the liquid, the pressure above its surface, and the intrinsic energy required to change the liquid into a gas.
The core principle is an energy exchange: evaporation accelerates when energy is added to a liquid more quickly than the surrounding environment can resist the escape of its molecules. Understanding how to manipulate heat, pressure, and surface area gives you direct control over this process.

The Core Drivers of Evaporation
To truly understand the rate of evaporation, we must look at the physical forces at play. These factors work in concert to either encourage or hinder the escape of molecules from a liquid's surface.
Heat Transfer: The Primary Engine
The most significant driver is the rate at which heat energy is supplied to the liquid. Heat increases the kinetic energy of the liquid's molecules.
When a molecule gains enough kinetic energy to overcome the intermolecular forces holding it to its neighbors, it escapes the surface as a gas. Therefore, a higher rate of heat transfer directly translates to a faster rate of evaporation.
Vapor Pressure and External Pressure: The Battle at the Surface
Every liquid exerts a vapor pressure, which is the force of its molecules pushing to escape into the gas phase. This is countered by the external pressure (usually atmospheric pressure) pushing down on the liquid's surface.
Evaporation occurs rapidly when the vapor pressure is high relative to the external pressure. This is why water evaporates faster at high altitudes where atmospheric pressure is lower.
Latent Heat of Vaporization: The Energy Cost
The latent heat of vaporization is the specific amount of energy required to convert one unit of a substance from liquid to gas without changing its temperature.
Substances with a high latent heat of vaporization, like water, require more energy input to evaporate each kilogram. This acts as a brake on the evaporation rate compared to liquids like alcohol, which have a lower energy cost.
Surface Area: The Gateway for Escape
Evaporation is a surface phenomenon. Only molecules at or near the surface can escape.
By increasing the surface area—for example, by spreading a puddle of water out—you expose more molecules to the air, creating more opportunities for them to escape. This dramatically increases the overall evaporation rate.
Environmental and Substance-Specific Factors
Beyond the core physics, the immediate environment and the liquid's composition add another layer of control.
Humidity and Airflow: Clearing the Path
The amount of vapor already in the surrounding air, known as humidity, affects the net rate of evaporation. If the air is already saturated, there is less "room" for new water molecules, slowing the process.
Airflow (wind) sweeps away the layer of humid air directly above the liquid's surface, replacing it with drier air. This maintains a steep concentration gradient and encourages more molecules to escape.
Composition of the Substance: The Internal Hurdles
The presence of solutes, like salt or sugar in water, can slow evaporation. These dissolved particles form bonds with water molecules, requiring more energy to break them apart.
This is why seawater evaporates more slowly than freshwater under identical conditions. The properties of the substance itself, and any changes it undergoes, are a critical factor.
Understanding the Trade-offs
Manipulating these factors often involves balancing competing priorities, especially in industrial or culinary applications.
The Link Between Pressure and Temperature
Reducing pressure lowers a liquid's boiling point. This powerful relationship allows for rapid evaporation at much lower temperatures than would otherwise be required.
This technique, known as vacuum evaporation, is essential for concentrating substances that are sensitive to heat, such as milk or fruit juices, without scorching or degrading them.
The Limit of Heat Input
While adding heat is the fastest way to increase evaporation, there is often a maximum allowable temperature.
Exceeding this limit can cause unwanted chemical reactions, burning, or decomposition of the product. The goal is often to find the highest possible heat transfer rate that doesn't compromise the substance's integrity.
How to Control Evaporation for Your Goal
Your strategy for managing evaporation depends entirely on your desired outcome.
- If your primary focus is speed: Maximize the rate of heat input, increase surface area, ensure constant airflow, and, if possible, reduce the ambient pressure.
- If your primary focus is preserving the substance: Use a lower temperature combined with reduced pressure to achieve evaporation without heat damage.
- If your primary focus is energy efficiency: Focus on optimizing heat transfer and managing airflow to remove saturated vapor, preventing wasted energy.
Ultimately, controlling evaporation is a process of managing the flow of energy into and out of a system.
Summary Table:
| Factor | Effect on Evaporation Rate | Key Principle |
|---|---|---|
| Heat Transfer Rate | Increases | Primary driver; adds kinetic energy to molecules. |
| Vapor Pressure vs. External Pressure | Increases when vapor pressure > external pressure | Molecules escape more easily when external pressure is lower. |
| Surface Area | Increases | More molecules are exposed at the surface, enabling escape. |
| Latent Heat of Vaporization | Decreases for substances with high latent heat | Higher energy cost per molecule slows the rate. |
| Humidity | Decreases | Saturated air reduces the concentration gradient for escape. |
| Airflow | Increases | Removes saturated vapor, maintaining a steep concentration gradient. |
Need precise control over evaporation in your lab processes? KINTEK specializes in high-performance lab equipment, including vacuum evaporators and heating systems designed to optimize heat transfer, pressure, and surface area for your specific applications—whether you're concentrating heat-sensitive samples or maximizing throughput. Let our experts help you select the right solution to enhance efficiency and preserve sample integrity. Contact KINTEK today to discuss your laboratory evaporation needs!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
People Also Ask
- What are the three stages of freeze drying? A Guide to Lyophilization for Lab Professionals
- Why is freeze drying a good method for preserving fruits and vegetables? Unlock Superior Food Preservation
- What happens during the primary drying phase of freeze drying? Master the Sublimation Process
- What are the factors affecting evaporation? Control the Rate of Any Liquid's Vaporization
- What are the main advantages of freeze drying? Achieve Superior Preservation for Sensitive Materials



















