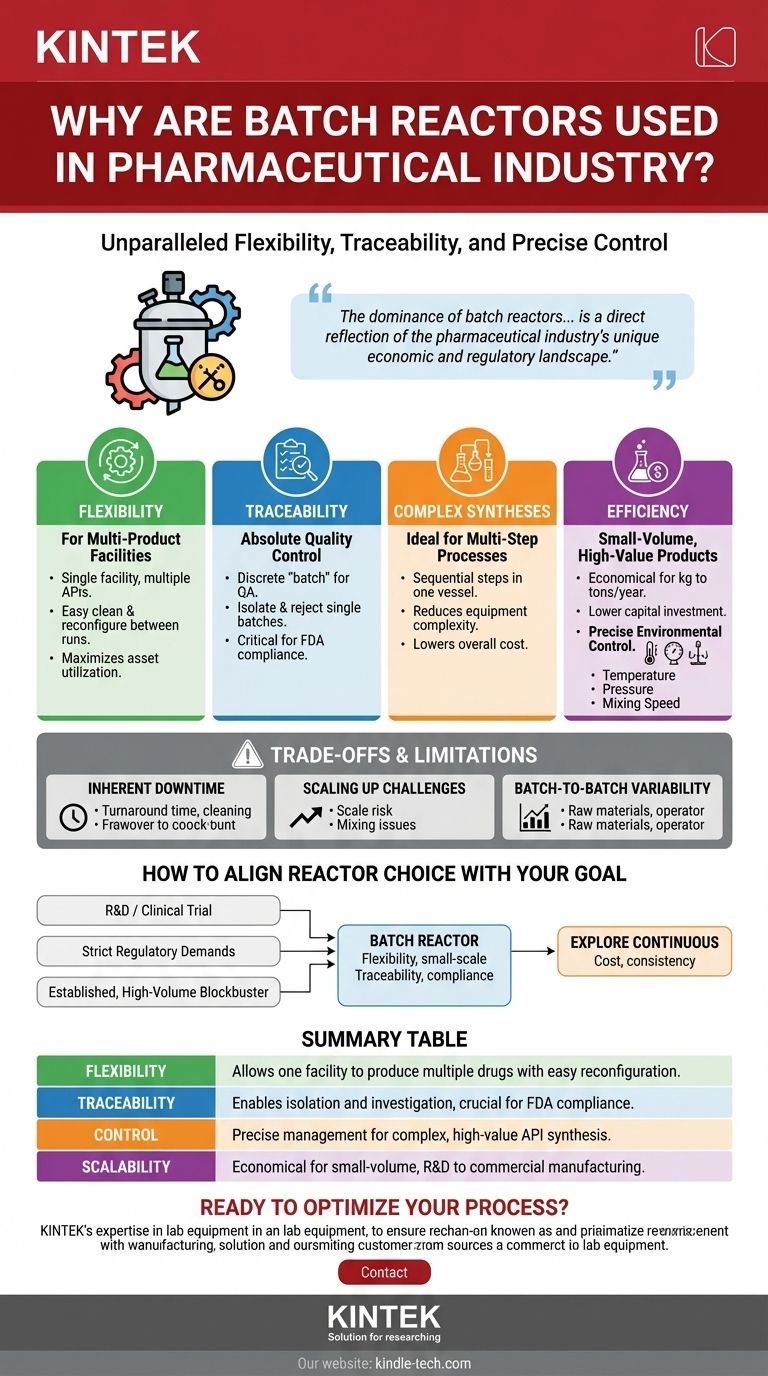
In the pharmaceutical industry, batch reactors are the default choice because they offer an unparalleled combination of flexibility, traceability, and precise control. This allows manufacturers to produce a wide variety of complex, high-value drugs in a single facility while adhering to the strictest quality and regulatory standards. Each batch is a discrete, controllable unit, which is perfectly suited to the industry's focus on quality over sheer quantity.
The dominance of batch reactors is not just a matter of tradition; it is a direct reflection of the pharmaceutical industry's unique economic and regulatory landscape. The need for absolute quality control, product versatility, and process traceability makes the batch model strategically superior to continuous production for most drug manufacturing scenarios.

The Core Advantages of Batch Processing in Pharma
The decision to use batch reactors is driven by several key factors that align perfectly with the goals of drug development and manufacturing.
Unmatched Flexibility for Multi-Product Facilities
Pharmaceutical plants rarely produce a single product. Batch reactors excel in this environment because they can be thoroughly cleaned and reconfigured between runs.
This allows a single facility to manufacture many different Active Pharmaceutical Ingredients (APIs) without the need for dedicated, single-product production lines, maximizing asset utilization.
Absolute Traceability and Quality Control
Traceability is non-negotiable in pharmaceutical manufacturing. The discrete nature of a "batch" provides a clear and contained unit for quality assurance.
If a deviation occurs, the entire batch can be isolated, investigated, and rejected without impacting any other products. This well-defined segregation is critical for meeting regulatory requirements from agencies like the FDA.
Ideal for Complex, Multi-Step Syntheses
Most modern drugs are the result of complex, multi-stage chemical syntheses. A single batch reactor can often be used to perform several sequential steps.
For example, the same vessel can be used for a reaction, a temperature change, the addition of a new reagent for a subsequent reaction, and finally, the initial stages of product isolation. This reduces equipment complexity and cost.
Efficient for Small-Volume, High-Value Products
Unlike commodity chemicals produced in massive quantities, many drugs are manufactured in relatively small volumes, from kilograms to a few metric tons per year.
The capital investment for a batch system is often more economical at this scale compared to a continuous processing plant, which requires significant upfront cost and is optimized for high, steady output.
Precise Environmental Control
Achieving the desired product purity and yield requires precise control over the reaction environment. Batch reactors are engineered for this level of precision.
Operators can meticulously manage critical parameters like temperature, pressure, and mixing speed throughout the entire reaction cycle. As noted in process studies, manipulating pressure can accelerate reaction kinetics and give chemists fine control over the formation of complex molecules.
Understanding the Trade-offs and Limitations
While dominant, the batch model is not without its challenges. Understanding these trade-offs is crucial for effective process management.
Inherent Downtime Between Batches
The primary drawback of batch processing is the unproductive time between cycles. This includes time spent discharging the product, cleaning the reactor, and setting up for the next run.
This "turnaround time" represents a significant portion of the total operational time and limits the maximum throughput of the facility.
Challenges with Scaling Up
A process that works perfectly in a 1-liter laboratory reactor may behave differently in a 5,000-liter production vessel.
Issues like inefficient mixing or inadequate heat transfer become more pronounced at larger scales. This "scale-up" risk requires extensive process engineering and validation to ensure consistency and safety.
Batch-to-Batch Variability
While a key goal is perfect consistency, slight variations between batches can and do occur. These can be caused by minor differences in raw material quality, operator actions, or equipment performance.
Managing and minimizing this batch-to-batch variability is a primary focus of process analytical technology (PAT) and statistical process control (SPC) in the pharmaceutical industry.
How to Align Reactor Choice with Your Goal
The right approach depends entirely on your specific position in the product lifecycle and your primary business objectives.
- If your primary focus is R&D or clinical trial manufacturing: The batch reactor is your unequivocal choice for its flexibility and suitability for small-scale, exploratory synthesis.
- If your primary focus is meeting strict regulatory demands: The inherent traceability of the batch model provides the most straightforward path to compliance and quality assurance.
- If your primary focus is producing an established, high-volume blockbuster drug: While batch processing is still the norm, this is the area where exploring continuous manufacturing may offer long-term cost and consistency benefits.
Ultimately, the batch reactor remains the cornerstone of pharmaceutical manufacturing because it provides a reliable and adaptable framework to balance the competing demands of chemistry, regulation, and economics.
Summary Table:
| Key Advantage | Why It Matters in Pharma |
|---|---|
| Flexibility | Allows one facility to produce multiple drugs with easy reconfiguration between batches. |
| Traceability | Enables isolation and investigation of any single batch, crucial for FDA and regulatory compliance. |
| Control | Precise management of temperature, pressure, and mixing for complex, high-value API synthesis. |
| Scalability | More economical for small-volume production, from R&D to commercial-scale manufacturing. |
Ready to Optimize Your Pharmaceutical Manufacturing Process?
Choosing the right reactor is critical to your success. KINTEK specializes in high-quality lab equipment and consumables, serving the precise needs of pharmaceutical laboratories. Whether you are scaling up a new API or require reliable equipment for R&D, our expertise can help you achieve superior control, traceability, and efficiency.
Contact our experts today to discuss how our solutions can support your drug development and manufacturing goals.
Visual Guide
Related Products
- 10L Chilling Circulator Cooling Water Bath Low Temperature Constant Temperature Reaction Bath
- 5L Chilling Circulator Cooling Water Bath Circulator for Low Temperature Constant Temperature Reaction Bath
- Shaking Incubators for Diverse Laboratory Applications
- Laboratory Oscillating Orbital Shaker
- Custom PTFE Teflon Parts Manufacturer for Reagent Wide Mouth Fine Mouth Sample High Temperature Bottles
People Also Ask
- Why is a circulating water chiller required for Prussian Blue nanoparticles? Ensure Stability & Batch Reproducibility
- Why is it necessary to equip corn cob hydrolysis systems with rapid cooling? Maximize Glucose and Xylose Yield
- Why is a high-performance cooling circulator necessary in silica membrane desalination? Boost Your Permeate Mass Transfer
- What is the importance of a Recirculating Cooling Water System? Protect Your Lab and Master Reaction Control
- What is the purpose of using a cooling water system after wheat straw pretreatment? Optimize Sugar Yield and Safety



















