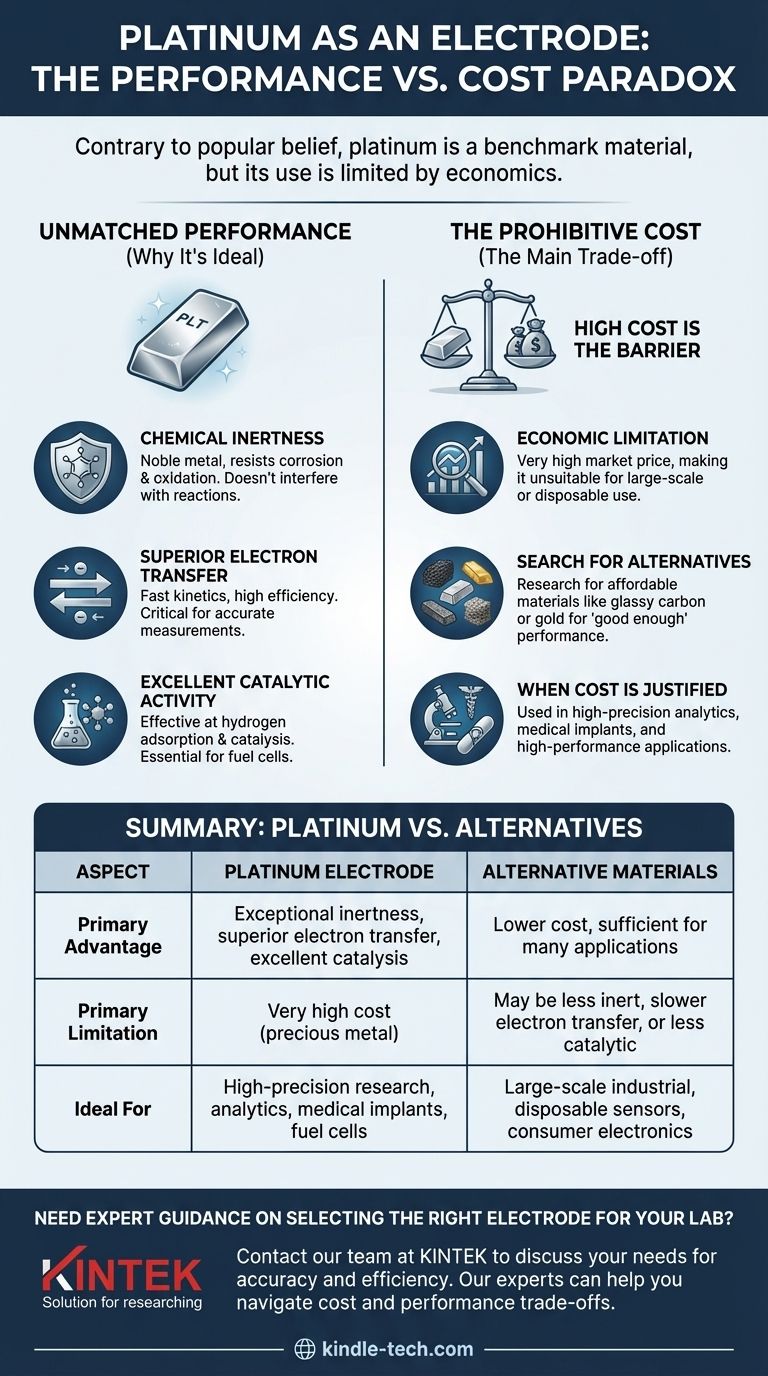
Contrary to the premise, platinum is one of the most widely used and effective electrode materials, especially in laboratory and high-performance applications. Its reputation is built on its exceptional chemical inertness and efficiency in electron transfer. The core confusion arises not from its performance, but from practical limitations that restrict its use in certain contexts.
The question is not why platinum isn't used as an electrode, but rather why its use is often limited to specific applications. The answer is overwhelmingly due to its high cost, not any lack of performance.

Why Platinum is a Benchmark Electrode Material
Platinum's properties make it a near-ideal material for conducting electrochemical experiments, serving as the standard against which many other materials are measured.
Unmatched Chemical Inertness
Platinum is a noble metal, meaning it strongly resists oxidation and corrosion. Unlike more reactive metals like iron or zinc, a platinum electrode does not participate in the chemical reaction being studied. This ensures that the measurements taken are a true reflection of the intended reaction, free from interference or contamination from the electrode itself.
Superior Electron Transfer
An electrode's primary job is to facilitate the movement of electrons between the external circuit and the chemical species in a solution. Platinum excels at this, offering very fast electron transfer kinetics. This efficiency is critical for accurate measurements and for driving reactions at a desirable rate.
Excellent Catalytic Activity
Beyond being a simple conductor, platinum often acts as a catalyst. It is particularly effective at absorbing hydrogen and catalyzing reactions involving hydrogen ions. This property is fundamental to its role in the Standard Hydrogen Electrode (SHE) and in hydrogen fuel cell technology.
Understanding the Trade-off: Performance vs. Cost
While technically superior in many ways, platinum's primary drawback is not chemical or electrical—it is economic.
The Prohibitive Cost
Platinum is a precious metal, and its market price is exceptionally high. This cost is the single greatest barrier to its widespread use in large-scale industrial processes, consumer products, or disposable sensors where cheaper alternatives are "good enough."
The Search for Alternatives
The high cost of platinum drives significant research into finding more affordable materials. For many applications, materials like glassy carbon, gold, graphite, and various metal oxides can provide adequate performance at a fraction of the cost.
When Cost Is Justified
In situations where precision, longevity, and reliability are non-negotiable, the cost of platinum is justified. This includes sensitive analytical instruments, medical implants where biocompatibility is crucial, and applications like high-performance fuel cells where its catalytic efficiency is unmatched.
Making the Right Choice for Your Application
Selecting an electrode material always involves balancing ideal electrochemical properties against real-world constraints.
- If your primary focus is high-precision research or analytics: Platinum is often the superior choice due to its inertness, ensuring the integrity and accuracy of your data.
- If your primary focus is a large-scale industrial process: The cost of platinum is likely prohibitive, making materials like stainless steel, graphite, or specialized alloys the only practical options.
- If your primary focus is building disposable sensors or consumer electronics: Cost-effective materials like screen-printed carbon or gold-plated contacts are the standard.
Ultimately, platinum's role as an electrode is defined by a trade-off between its exceptional performance and its significant cost.
Summary Table:
| Aspect | Platinum Electrode | Alternative Materials |
|---|---|---|
| Primary Advantage | Exceptional chemical inertness, superior electron transfer, excellent catalytic activity | Lower cost, sufficient for many applications |
| Primary Limitation | Very high cost (precious metal) | May be less inert, slower electron transfer, or less catalytic |
| Ideal For | High-precision lab research, analytical instruments, medical implants, fuel cells | Large-scale industrial processes, disposable sensors, consumer electronics |
Need expert guidance on selecting the right electrode material for your specific laboratory application?
At KINTEK, we specialize in providing high-performance lab equipment and consumables. Our experts can help you navigate the trade-offs between cost and performance to select the ideal electrode for your research or process, whether it requires the unparalleled quality of platinum or a more cost-effective alternative.
Contact our team today to discuss your needs and ensure your lab achieves the highest levels of accuracy and efficiency.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements



















