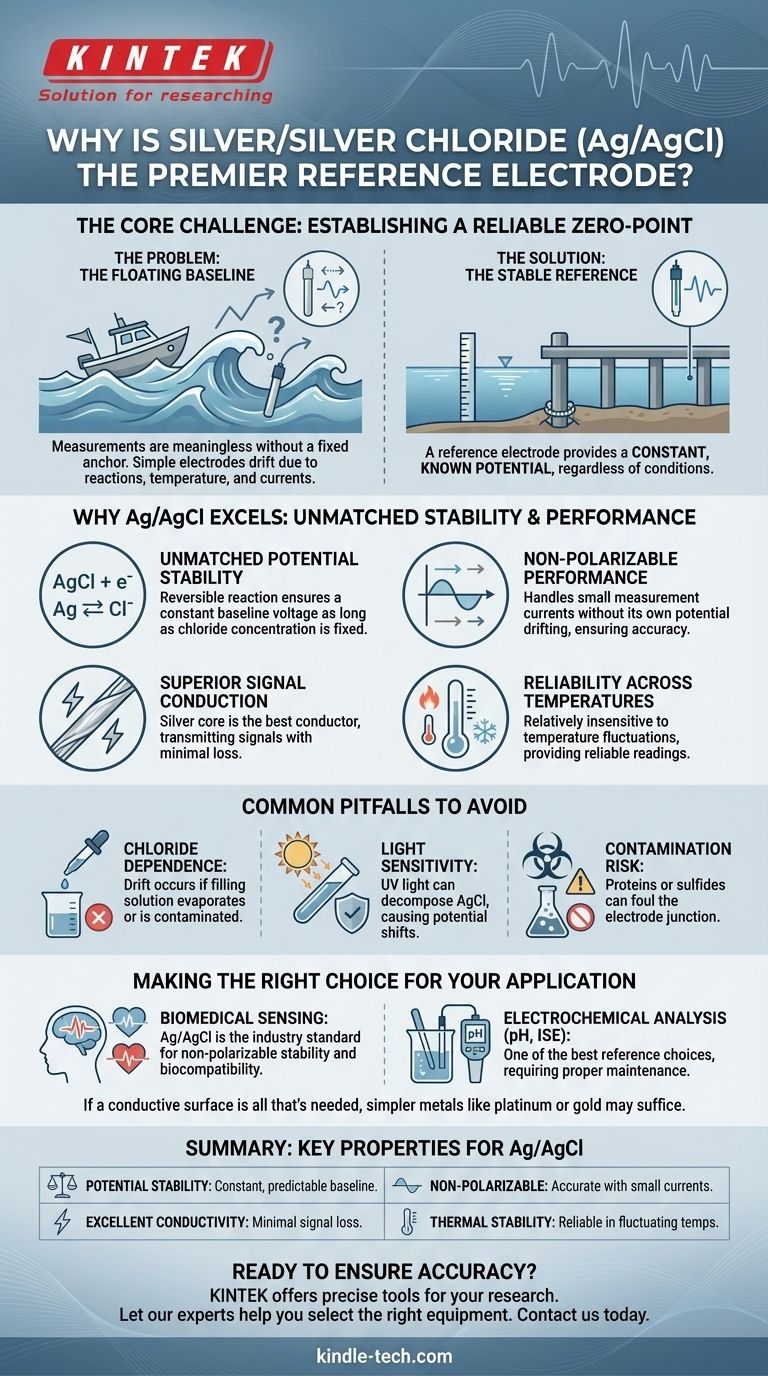
The primary reason silver/silver chloride (Ag/AgCl) is so useful is not just because silver is an excellent conductor, but because the Ag/AgCl combination creates an exceptionally stable and predictable electrochemical benchmark. This stability makes it a premier choice for a reference electrode, which is essential for accurate voltage measurements in countless scientific and medical applications.
The core challenge in measuring any electrical potential is establishing a reliable, unwavering zero-point. The silver/silver chloride electrode excels because its specific chemical properties provide this stable baseline, acting like a fixed anchor in a fluctuating sea of electrical signals.
The Foundation: What Makes a "Good" Electrode?
To understand the value of Ag/AgCl, we first have to understand the fundamental problem it solves. An electrode's job is often to measure a voltage, but voltage is a relative value—it can only be measured between two points.
The Problem of the Floating Baseline
Imagine trying to measure the height of a small wave while you are on a boat that is also rising and falling with larger swells. Your measurements would be meaningless because your reference point is constantly moving.
Many simple metal electrodes behave like that boat. Their own electrical potential can shift due to chemical reactions, temperature changes, or the flow of small currents. This makes them unsuitable for sensitive measurements.
The Role of a Reference Electrode
A high-quality reference electrode is the solution. Its purpose is to provide a constant, known potential that does not change, regardless of the conditions in the solution it's measuring.
This stable reference acts like a fixed pier next to the ocean. By measuring the water level from that pier, you can get an accurate reading of the waves, confident that your baseline isn't moving.
Why Silver/Silver Chloride Excels
The Ag/AgCl system has a unique combination of properties that make it an almost ideal reference electrode.
Unmatched Potential Stability
The true genius of the Ag/AgCl electrode is its reversible chemical reaction: AgCl + e⁻ ⇌ Ag + Cl⁻.
The potential of the electrode is determined by the concentration of chloride ions (Cl⁻) in the surrounding electrolyte. As long as this concentration is kept constant, the electrode's voltage is incredibly stable and predictable.
Non-Polarizable Performance
A "non-polarizable" electrode is one whose potential does not change when a small amount of current flows through it.
Because the chemical reaction is so efficient and reversible, the Ag/AgCl electrode can handle the small currents required for measurement without its own baseline potential drifting. This is critical for obtaining accurate data.
Superior Signal Conduction
This is where the properties of pure silver come into play. As the best electrical conductor among all metals, silver ensures that the measured signal is transmitted from the electrode to the measurement instrument with minimal resistance or loss.
Reliability Across Temperatures
As noted, the Ag/AgCl electrode is relatively insensitive to changes in temperature. This thermal stability means it can be trusted to provide reliable readings in environments that are not perfectly temperature-controlled, a common scenario in both laboratory and real-world settings.
Common Pitfalls to Avoid
While Ag/AgCl is a superior reference electrode, it is not without limitations that require careful handling.
Dependence on Chloride Concentration
The electrode's stability is entirely dependent on a constant chloride ion concentration in the filling solution. If this solution becomes contaminated or evaporates, the reference potential will drift, destroying measurement accuracy.
Light Sensitivity
Ag/AgCl electrodes are sensitive to light, particularly UV light, which can decompose the silver chloride and cause the potential to shift. For this reason, they are often stored in the dark or housed in amber-colored glass.
Contamination Risk
In certain applications, particularly biological ones, substances like proteins or sulfides can foul the electrode junction. This contamination can interfere with the chemical equilibrium and compromise the stability of the reference potential.
Making the Right Choice for Your Application
Understanding these principles allows you to select the right tool for the job.
- If your primary focus is biomedical sensing (ECG, EEG, EMG): Ag/AgCl is the undisputed industry standard due to its non-polarizable nature, stability, and excellent biocompatibility.
- If your primary focus is precise electrochemical analysis (pH, ion-selective measurements): Ag/AgCl is one of the best reference electrodes you can choose, provided you properly maintain the filling solution and protect it from light and contaminants.
- If you simply need a conductive surface (a working electrode): The stability of Ag/AgCl is unnecessary. Simpler, more inert materials like platinum, gold, or glassy carbon are often better choices.
Ultimately, choosing the right electrode begins with understanding that a stable reference is the bedrock of any accurate electrical measurement.
Summary Table:
| Key Property | Why It Matters for Ag/AgCl |
|---|---|
| Potential Stability | Reversible reaction provides a constant, predictable baseline voltage. |
| Non-Polarizable | Handles small currents without drifting, ensuring measurement accuracy. |
| Excellent Conductivity | Silver core transmits signals with minimal loss. |
| Thermal Stability | Provides reliable readings even with temperature fluctuations. |
Ready to ensure the accuracy of your electrochemical or biomedical measurements?
The stable baseline provided by a high-quality reference electrode is critical for reliable data. KINTEK specializes in laboratory equipment and consumables, offering the precise tools you need for your research and analysis.
Let our experts help you select the right equipment for your application. Contact us today to discuss your specific needs and discover how we can support your laboratory's success.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What are the characteristics of a non-aqueous silver ion electrode? A Guide to Stable Potentials in Organic Solvents
- What are the key properties and applications of glassy carbon electrodes? | Your Guide to Superior Electrochemical Analysis
- What role does a tungsten electrode play in monitoring MgOH+ in molten salts? Expert Cyclic Voltammetry Insights
- Why are large-area Platinum foils or Graphite rods selected as counter electrodes? Ensure Precise Corrosion Research
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is the role of the Platinum electrode in Zircaloy-2 testing? Ensure High-Purity Electrochemical Results
- What is the role of the Pt mesh and Ag/AgCl electrode? Optimize Your Three-Electrode Electrochemical System
- What is the function of a laboratory drying oven in Ag-TiO2 sol-coating? Fixation and Precision for Electrodes



















