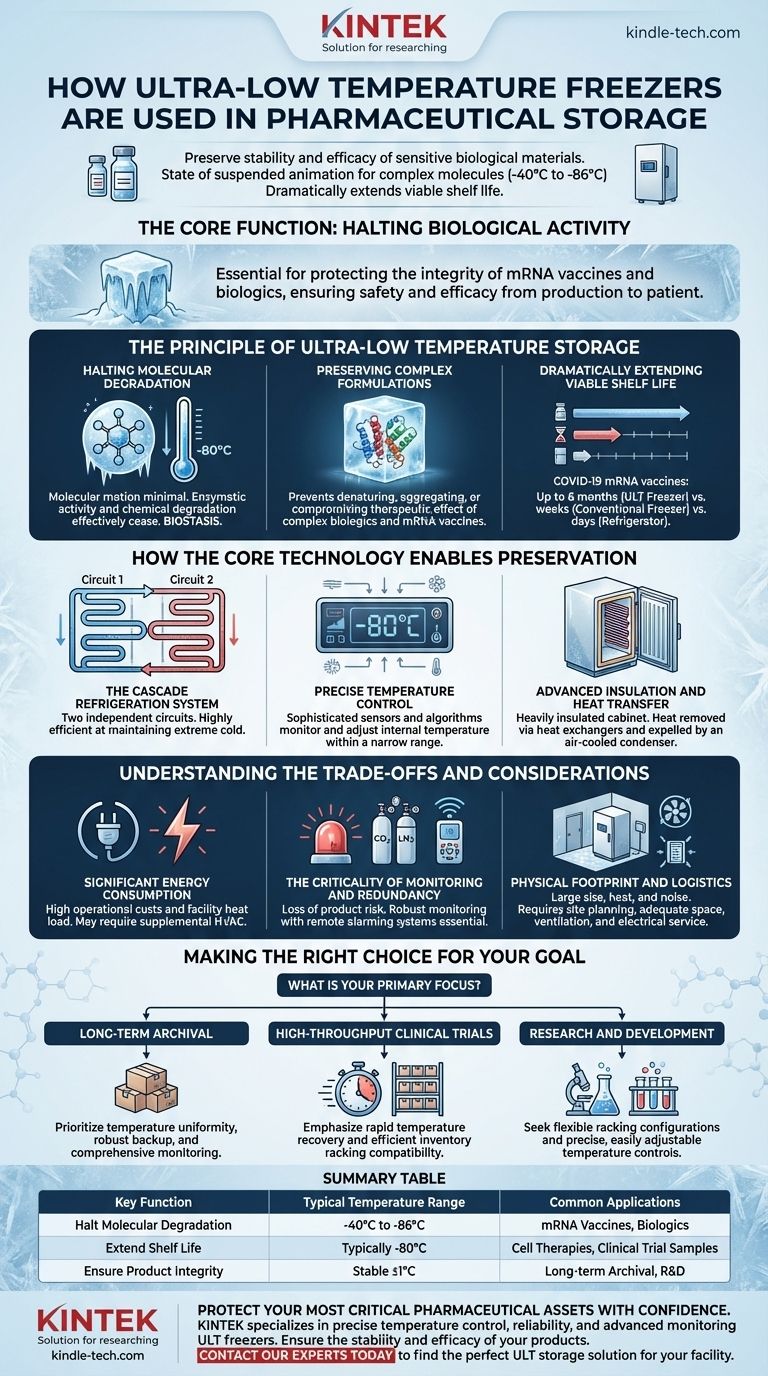
In pharmaceuticals, ultra-low temperature (ULT) freezers are used to preserve the stability and efficacy of sensitive biological materials that would otherwise degrade at standard freezer temperatures. They provide a state of suspended animation for complex molecules by storing products like vaccines, cell therapies, and clinical trial samples at temperatures ranging from -40°C to -86°C, dramatically extending their viable shelf life.
The core function of a ULT freezer in pharmaceuticals is not just cold storage; it is to halt biological and chemical activity at a molecular level. This capability is essential for protecting the integrity of high-value, temperature-sensitive products like mRNA vaccines and biologics, ensuring they remain safe and effective from production to patient.

The Principle of Ultra-Low Temperature Storage
To understand the role of ULT freezers, you must first understand why such extreme temperatures are necessary. It goes beyond simple preservation and into the realm of molecular biophysics.
Halting Molecular Degradation
At standard refrigeration or even freezer temperatures, enzymatic and chemical reactions continue, albeit at a slower pace. For highly complex molecules like RNA or therapeutic proteins, even this slow degradation can render a product useless.
ULT freezers push temperatures down to a point (typically -80°C) where molecular motion is so minimal that enzymatic activity and chemical degradation effectively cease. This creates a state of biostasis, preserving the intricate structure of the biological material.
Preserving Complex Formulations
Modern pharmaceuticals, particularly biologics and mRNA vaccines, are not simple chemical compounds. They are complex, three-dimensional structures suspended in specific formulations.
Extreme cold locks these structures in place, preventing them from denaturing, aggregating, or interacting in ways that would compromise their therapeutic effect.
Dramatically Extending Viable Shelf Life
The difference in preservation is not minor. For example, certain COVID-19 mRNA vaccines can be stored for up to six months in a ULT freezer.
The same vaccine might only last for a few weeks in a conventional laboratory freezer and mere days in a refrigerator. This extended shelf life is critical for global distribution and inventory management.
How the Core Technology Enables Preservation
ULT freezers achieve these extreme temperatures using specialized engineering that distinguishes them from standard refrigeration units.
The Cascade Refrigeration System
Most ULT freezers use a cascade system involving two independent refrigeration circuits. The first circuit cools the second, allowing the second circuit to reach a much lower temperature than a single compressor could achieve alone.
This two-stage process is highly efficient at extracting heat and maintaining the extreme cold necessary for long-term storage.
Precise Temperature Control
These units are not simply "on" or "off." They employ sophisticated sensors, feedback loops, and control algorithms to constantly monitor and adjust the internal temperature.
This ensures the temperature remains stable within a very narrow range of the setpoint, preventing fluctuations that could damage sensitive samples.
Advanced Insulation and Heat Transfer
The cabinet is heavily insulated to prevent ambient heat from entering. Internally, heat is removed from the chamber via steel plate heat exchangers or coils. An air-cooled condenser then expels this heat into the surrounding room.
Understanding the Trade-offs and Considerations
While essential, deploying ULT freezers requires careful planning and an understanding of their operational demands.
Significant Energy Consumption
A cascade refrigeration system is powerful and consumes a substantial amount of electricity. This contributes to both operational costs and the heat load of the facility, which may require supplemental HVAC capacity.
The Criticality of Monitoring and Redundancy
A freezer failure can result in the loss of millions of dollars worth of product or irreplaceable research samples.
Therefore, robust temperature monitoring with remote alarming is not optional; it is a fundamental requirement. Many facilities also use backup systems, such as CO2 or liquid nitrogen (LN2) injection, that automatically deploy if the primary system fails.
Physical Footprint and Logistics
ULT freezers are large, heavy, and generate significant heat and noise. Proper site planning is essential to ensure adequate space, floor support, ventilation, and electrical service. Effective inventory management systems are also crucial for tracking samples within the freezer.
Making the Right Choice for Your Goal
Selecting and implementing ULT storage solutions depends entirely on the specific application and the assets you need to protect.
- If your primary focus is long-term archival of high-value biologics: Prioritize freezers with proven temperature uniformity, robust backup systems (like CO2/LN2), and comprehensive monitoring.
- If your primary focus is high-throughput clinical trial management: Emphasize models with rapid temperature recovery after door openings and compatibility with efficient inventory racking systems.
- If your primary focus is research and development: Seek units that offer flexible racking configurations and precise, easily adjustable temperature controls to accommodate a variety of sample types.
Ultimately, investing in the right ultra-low temperature storage strategy is a direct investment in protecting your most critical pharmaceutical assets.
Summary Table:
| Key Function | Typical Temperature Range | Common Applications |
|---|---|---|
| Halt Molecular Degradation | -40°C to -86°C | mRNA Vaccines, Biologics |
| Extend Shelf Life | Typically -80°C | Cell Therapies, Clinical Trial Samples |
| Ensure Product Integrity | Stable ±1°C | Long-term Archival, R&D |
Protect your most critical pharmaceutical assets with confidence. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our ultra-low temperature freezers are engineered for precise temperature control, reliability, and advanced monitoring to safeguard your high-value biologics, vaccines, and research samples. Ensure the stability and efficacy of your products—contact our experts today to find the perfect ULT storage solution for your facility.
Visual Guide
Related Products
- 108L Vertical Ultra Low Temperature ULT Freezer
- 808L Precision Laboratory Vertical Ultra Low Temperature Freezer
- 158L Precision Vertical Ultra Low Freezer for Laboratory Applications
- 708L Ultra Low Temperature Freezer High Performance Laboratory Freezer
- 208L Advanced Precision Laboratory Ultra Low Temperature Freezer for Cold Storage
People Also Ask
- How does fast temperature recovery benefit ultra-low freezers? Protect Sample Integrity and Lab Efficiency
- What is the purpose of ultra-low temperature (ULT) freezers? Preserve Critical Biological Samples
- What are ultra-low temperature freezers designed for? Preserving Your Most Valuable Biological Samples
- How do ultra-low temperature freezers work? Unlocking the Secrets of -86°C Sample Preservation
- How is temperature controlled in ultra low temperature freezers? A Guide to Stable -80°C Storage



















