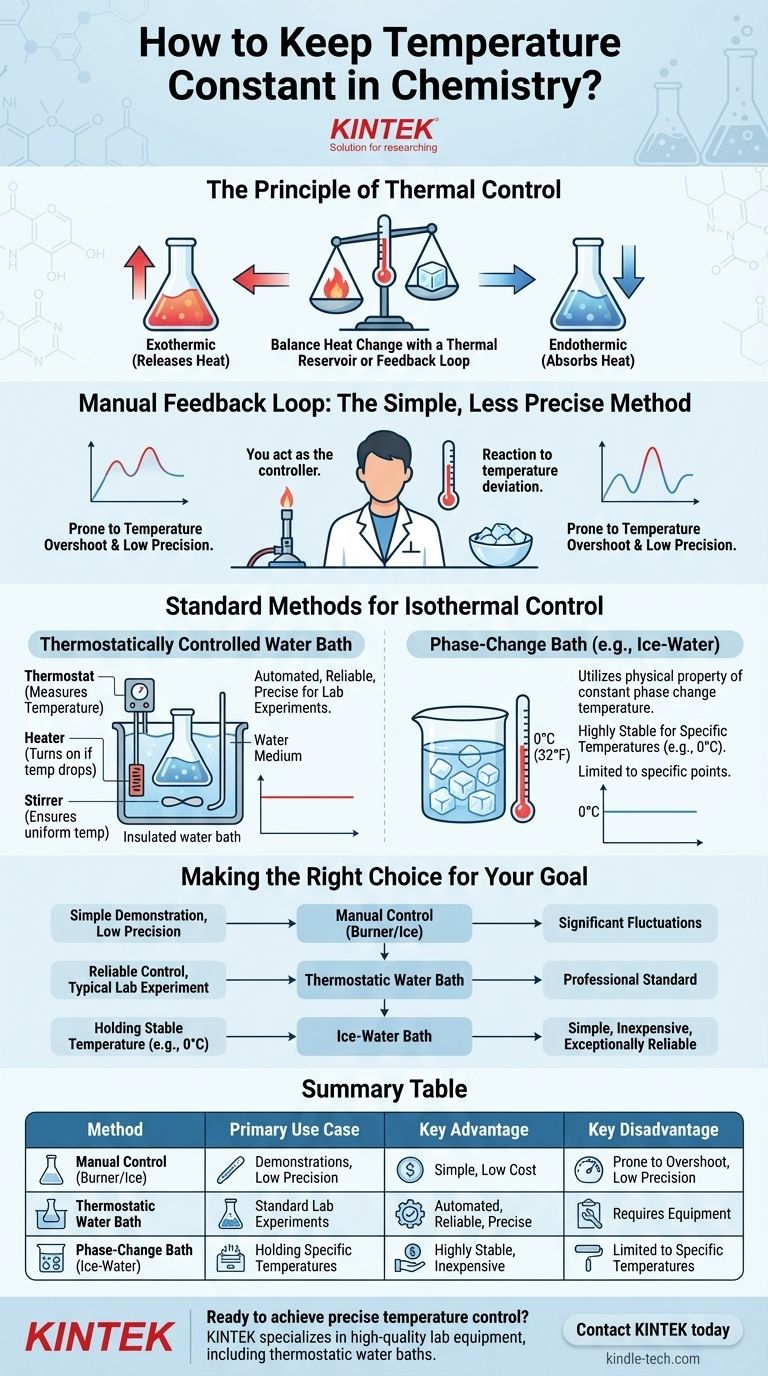
To maintain a constant temperature in chemistry, you must create a system that can add or remove heat as needed to counteract any changes. This is often done using a simple manual method, like adding ice to cool a solution or using a Bunsen burner to heat it, but professional labs rely on automated systems like water baths for precise control.
The core principle of maintaining a constant temperature (an isothermal condition) is using a thermal reservoir or a feedback loop. While manual adjustments are possible, automated systems that continuously measure and correct temperature are the standard for achieving reliable and precise results.

The Principle of Thermal Control
Any chemical reaction will either release heat (exothermic) or absorb heat (endothermic). To keep the temperature constant, you must have a mechanism to counteract this change, effectively pulling heat out of or adding heat into the system at the same rate.
The Manual Feedback Loop
The simplest method involves direct manual intervention based on observation. This creates a basic feedback loop where you are the controller.
The reference method of using a Bunsen burner and ice is a classic example. You monitor a thermometer and react to any deviation from your target temperature.
If the temperature drops, you briefly apply heat with the burner. If the temperature rises, you add a piece of ice.
The Challenge with Manual Control
This manual approach is often used in introductory settings but is rarely suitable for serious experimental work due to its significant drawbacks.
The primary issue is temperature overshoot. It is very easy to add too much heat or too much ice, causing the temperature to swing wildly above and below your target.
This method also requires constant, undivided attention and lacks the precision needed to study temperature-sensitive processes like reaction kinetics or equilibrium.
Standard Methods for Isothermal Control
To overcome the limitations of manual control, chemists use systems that provide a large, stable thermal environment or employ automated feedback loops.
The Thermostatically Controlled Water Bath
This is the most common and effective solution in a laboratory setting. A water bath is an insulated container of water with a built-in heater, stirrer, and thermostat.
The thermostat continuously measures the water temperature. If it drops, the heater turns on. If it gets too high, the heater turns off, allowing the water to cool slightly. The stirrer ensures the temperature is uniform throughout the bath.
By placing your reaction vessel inside this bath, you ensure it is surrounded by a medium of stable, constant temperature.
The Phase-Change Bath
A phase change, such as ice melting or water boiling, occurs at a constant temperature. This physical property can be used to create a highly stable thermal environment.
An ice-water bath is the most common example. A properly made mixture of ice and liquid water will hold a stable temperature of 0°C (32°F). It's crucial to have both ice and water to ensure good thermal contact with the reaction vessel.
Similarly, a boiling water bath will maintain a constant temperature of 100°C (212°F), though this is dependent on atmospheric pressure.
Making the Right Choice for Your Goal
Selecting the right method depends entirely on the precision your experiment requires.
- If your primary focus is a simple demonstration with low precision: The manual burner-and-ice method can illustrate the concept of temperature control, but be prepared for significant fluctuations.
- If your primary focus is reliable control for a typical lab experiment: A thermostatically controlled water bath is the professional standard and the most practical choice.
- If your primary focus is holding a stable temperature at exactly 0°C: A properly prepared ice-water bath is simple, inexpensive, and exceptionally reliable.
Ultimately, effective temperature control is about moving from manual reaction to an automated, stable system that manages heat flow for you.
Summary Table:
| Method | Primary Use Case | Key Advantage | Key Disadvantage |
|---|---|---|---|
| Manual Control (Burner/Ice) | Demonstrations, Low Precision | Simple, Low Cost | Prone to Overshoot, Low Precision |
| Thermostatic Water Bath | Standard Lab Experiments | Automated, Reliable, Precise | Requires Equipment |
| Phase-Change Bath (e.g., Ice-Water) | Holding Specific Temperatures (e.g., 0°C) | Highly Stable, Inexpensive | Limited to Specific Temperatures |
Ready to achieve precise temperature control in your lab? KINTEK specializes in high-quality lab equipment, including reliable thermostatic water baths and other temperature control solutions designed for the accuracy your chemistry experiments demand. Let our experts help you select the perfect equipment for your specific needs. Contact KINTEK today to enhance your lab's capabilities!
Visual Guide
Related Products
- 80L Chilling Circulator Cooling Water Circulator for Water Bath Cooling and Low Temperature Constant Temperature Reaction Bath
- 100L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 5L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 10L Chilling Circulator Cooling Water Bath Low Temperature Constant Temperature Reaction Bath
- 50L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
People Also Ask
- What is the function of a constant temperature heating and stirring device? Precision Control in Cr2O3 Nanoparticle Synthesis
- How to maintain temperature in a lab? Build a Stable, Multi-Layered Control System
- What is the purpose of an immersion chilling accessory? Expand Lab Flexibility and Thermal Range
- How do circulating cooling systems ensure accuracy in adsorption tests? Stabilize Thermal Variables for Precise Data
- What is the primary role of a constant temperature water bath in biomass washing? Optimize Poplar Pretreatment.
- What is the function of a constant temperature water bath? Optimize MgAl2O4 Precursor Homogeneity in Sol-Gel Methods
- How to keep a lab water bath clean? A Proactive Guide to Prevent Contamination & Scale
- What function does a laboratory shaking incubator serve for halophilic archaea? Optimize Your Seed Cultivation Today



















