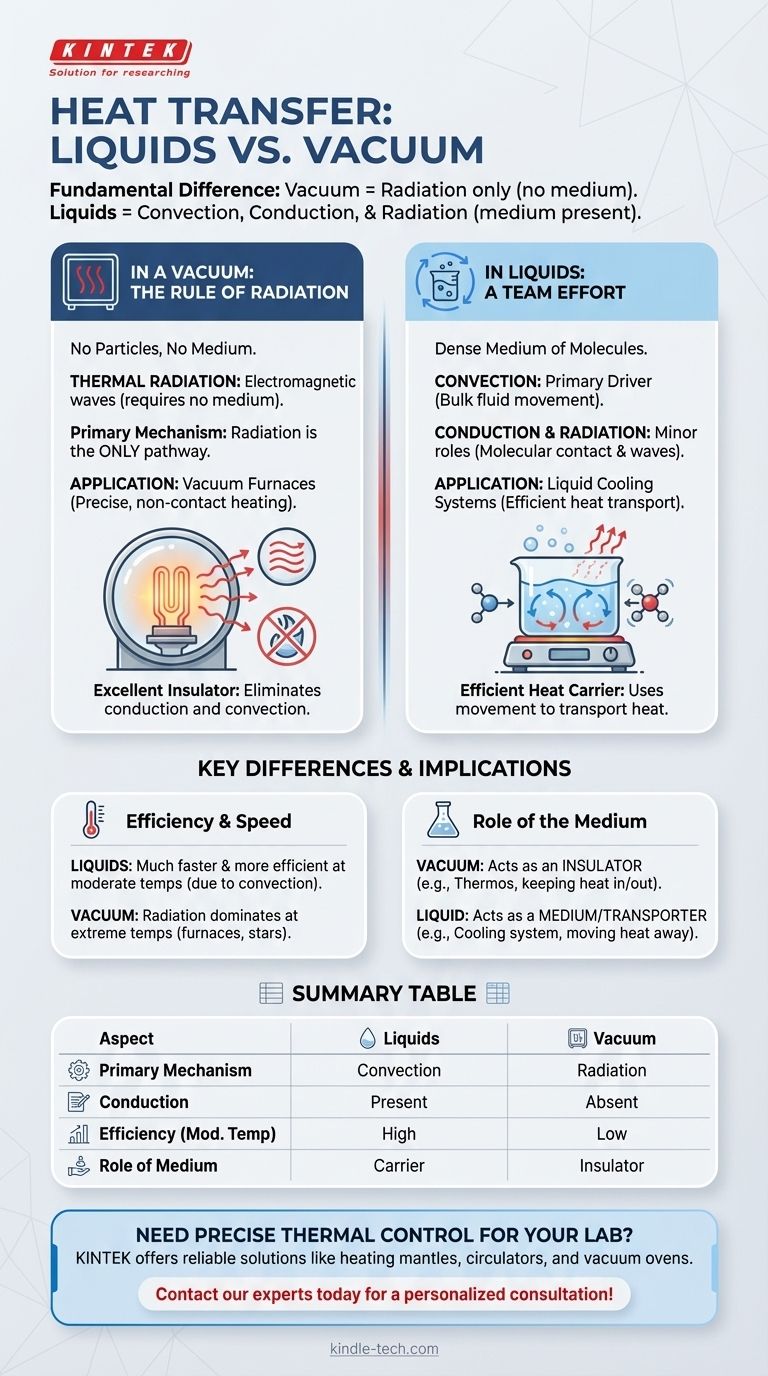
In essence, the fundamental difference between heat transfer in liquids and a vacuum lies in the mechanisms available for energy to travel. A vacuum, being devoid of matter, permits heat transfer exclusively through radiation. Liquids, on the other hand, utilize a combination of convection, conduction, and radiation, with convection typically being the most dominant mechanism.
The presence or absence of a physical medium is the single most important factor. A vacuum eliminates conduction and convection, making it an excellent insulator, while a liquid acts as a carrier, using its own movement to efficiently transport heat.

Heat Transfer in a Vacuum: The Rule of Radiation
In the emptiness of a vacuum, there are no particles to collide or flow. This leaves only one pathway for thermal energy to move from one place to another.
What is Thermal Radiation?
Thermal radiation is energy transferred in the form of electromagnetic waves, similar to light or radio waves. It does not require any medium to propagate.
Any object with a temperature above absolute zero emits this radiation. The hotter the object, the more energy it radiates. This is how the sun's heat travels through the vacuum of space to reach Earth.
No Medium Required
This is the defining characteristic of radiative heat transfer. It can move through a perfect vacuum unimpeded, which is impossible for other forms of heat transfer.
Application: Vacuum Furnaces
In industrial processes like vacuum induction sintering, this principle is used for precise heating. By removing air, heat transfer by convection is minimized, forcing radiation from a heating element to be the primary method. This allows for uniform, non-contact heating of a material's surface.
Heat Transfer in Liquids: A Team Effort
Unlike a vacuum, a liquid is a dense medium of molecules. This allows for two additional, and often far more effective, methods of heat transfer.
Convection: The Primary Driver
Convection is heat transfer through the bulk movement of a fluid. When a part of a liquid is heated, it expands, becomes less dense, and rises.
Cooler, denser liquid then moves in to take its place, gets heated, and also rises. This creates a continuous circulation, called a convection current, that efficiently distributes heat throughout the liquid. This is the main principle behind boiling a pot of water.
Conduction: The Molecular Hand-off
Conduction is the transfer of heat through direct molecular contact. Vibrating (hot) molecules bump into their neighbors, transferring kinetic energy to them.
While conduction occurs in liquids, its effect is often overshadowed by the much faster and large-scale energy transport provided by convection.
The Minor Role of Radiation
Radiation still occurs in liquids. Heat can be radiated from the surface of the liquid, and some radiation can travel through the liquid itself, though it is often absorbed quickly. In most common scenarios, however, its contribution is small compared to convection.
Understanding the Key Differences
The practical implications of these distinct mechanisms are significant, defining their use in engineering and science.
Efficiency and Speed
At everyday temperatures, convection in liquids is a much faster and more efficient way to transfer heat than radiation alone. This is why liquid cooling systems are so effective.
Radiation's effectiveness, however, increases dramatically with temperature. In the extreme heat of a furnace or a star, radiation becomes the dominant mode of heat transfer.
The Role of the Medium
A vacuum is an excellent insulator against conduction and convection. This is the principle behind vacuum flasks (like a Thermos), which use a vacuum layer to keep liquids hot or cold.
A liquid, conversely, is a medium for heat transfer. Its purpose in a cooling system is to absorb heat in one place and physically transport it somewhere else.
Making the Right Choice for Your Goal
Understanding these mechanisms allows you to control thermal energy for a specific purpose.
- If your primary focus is insulation or high-temperature processing: A vacuum is your tool. It eliminates conduction and convection, allowing you to either trap heat or control it precisely using radiation.
- If your primary focus is rapid cooling or heat distribution: A liquid is your solution. You must engineer a system that promotes strong convection currents to carry heat away quickly and efficiently.
Ultimately, mastering thermal management begins with understanding how the chosen medium—or the lack thereof—dictates the rules of energy transfer.
Summary Table:
| Aspect | Heat Transfer in Liquids | Heat Transfer in a Vacuum |
|---|---|---|
| Primary Mechanism | Convection (bulk fluid movement) | Radiation (electromagnetic waves) |
| Conduction | Present (molecular contact) | Absent (no medium) |
| Efficiency at Moderate Temperatures | High (due to convection) | Low |
| Role of Medium | Acts as a heat carrier | Acts as an insulator |
| Common Applications | Liquid cooling systems, heating baths | Vacuum furnaces, thermal insulation |
Need precise thermal control for your laboratory processes?
Whether your application requires the rapid, uniform heating of a liquid bath or the contamination-free, high-temperature environment of a vacuum furnace, KINTEK has the expertise and equipment to meet your needs. Our range of lab equipment, including heating mantles, circulators, and vacuum ovens, is designed to deliver reliable performance and exact temperature control.
Let us help you select the right solution for your specific thermal management challenges. Contact our experts today for a personalized consultation!
Visual Guide
Related Products
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
People Also Ask
- Why are vacuum furnaces or high-purity inert atmosphere furnaces required for joining refractory metals?
- How does the isothermal holding function of high-temperature furnaces affect TLP bonding joints? Achieve Seamless Bonds
- What industry is annealing used in? From Automotive to Medical Devices
- Are brazed joints stronger than welded joints? Choosing the Right Joining Method for Your Assembly
- What is the benefits of vacuum hardening? Achieve Superior Metallurgical Quality and Process Control
- What role does a vacuum heat treatment furnace play in the final processing of Nb-Ti alloy powders? Restoring Ductility
- Should I use flux when brazing aluminum? The Critical Role of Flux in Achieving a Strong Bond
- How does a vacuum affect heat transfer? Master Precise Thermal Control in Your Lab



















