In short, X-Ray Fluorescence (XRF) is both a qualitative and a quantitative analytical technique. Its function depends entirely on the analytical goal, the instrument's setup, and the methodology used. While every XRF measurement inherently provides qualitative data, achieving accurate quantitative results requires a more deliberate and rigorous process.
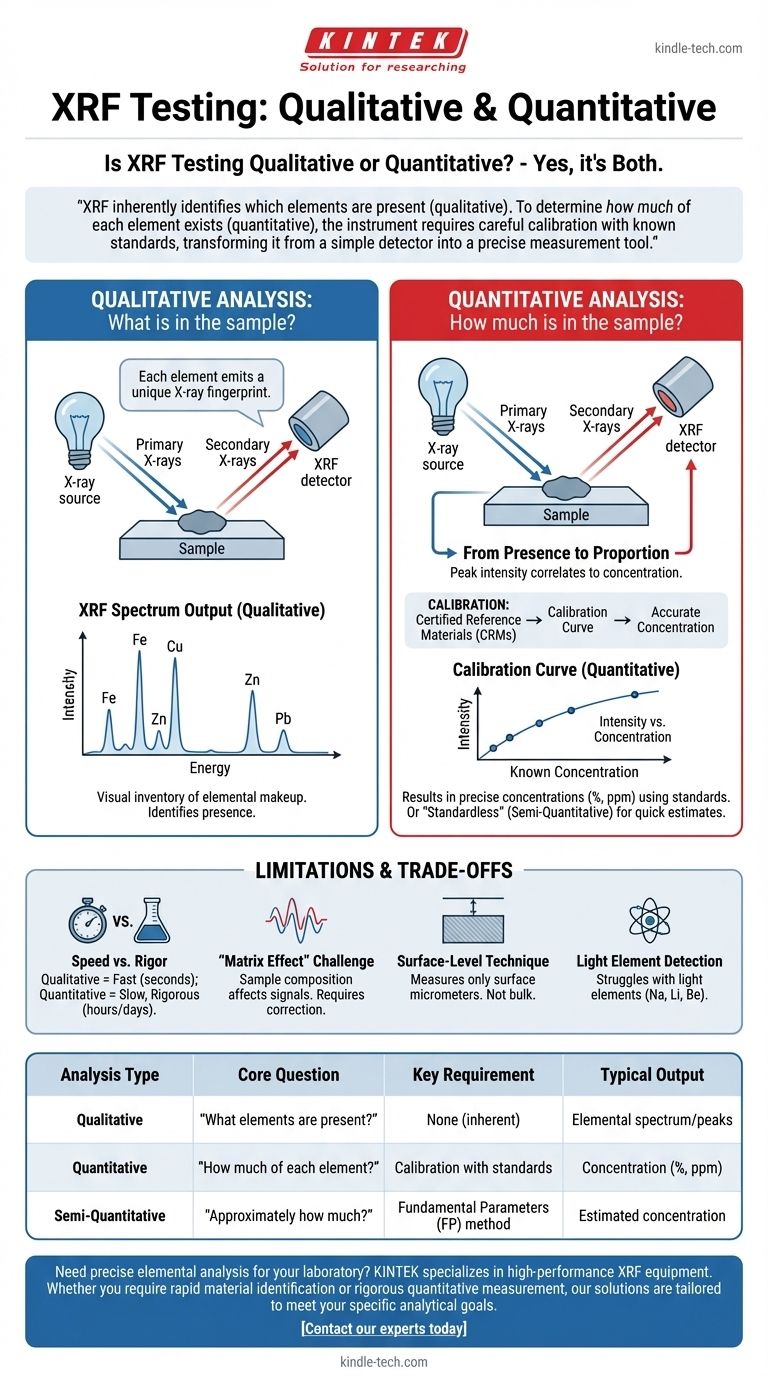
XRF inherently identifies which elements are present (qualitative). To determine how much of each element exists (quantitative), the instrument requires careful calibration with known standards, transforming it from a simple detector into a precise measurement tool.

How XRF Delivers Qualitative Analysis
Qualitative analysis answers the simple question: "What is in this sample?" This is the foundational capability of all XRF analyzers.
The Fundamental Principle: Identifying Elements
Every element, when energized by a primary X-ray source, emits its own unique set of secondary X-rays. These secondary X-rays have specific energy levels that act as an elemental fingerprint.
The XRF instrument's detector measures the energy of each X-ray it receives from the sample. By identifying these characteristic energies, the software can definitively determine which elements are present.
What Qualitative Results Look Like
The raw output is typically a spectrum, which is a graph showing X-ray intensity versus energy. Each peak on this graph corresponds to the unique energy fingerprint of a specific element, providing a clear, visual inventory of the sample's elemental makeup.
The Path to Quantitative Analysis
Quantitative analysis goes a step further to answer: "How much of each element is in this sample?" This requires converting the qualitative data into concentrations.
From Presence to Proportion
The intensity of an element's characteristic X-ray signal—essentially, the height of its peak on the spectrum—correlates directly to its concentration in the sample. A stronger signal generally means more of that element is present.
However, this relationship is not perfectly linear and can be influenced by other factors within the sample.
The Critical Role of Calibration
To achieve true quantitative results, the instrument must be calibrated. This involves measuring certified reference materials (CRMs) or "standards" that have a known and verified concentration of the elements you wish to measure.
By comparing the signal intensity from the unknown sample to the signal intensities from the known standards, the software can build a calibration curve. This curve allows it to accurately calculate the elemental concentrations in your sample, often expressed as a percentage or parts per million (PPM).
"Standardless" Analysis
Some XRF systems offer "standardless" or "fundamental parameters" (FP) analysis. This method uses theoretical physics principles and algorithms to estimate concentrations without direct calibration standards. While incredibly useful for quick estimates, it is generally considered semi-quantitative and is less accurate than methods using sample-specific calibrations.
Understanding the Trade-offs and Limitations
While powerful, XRF is not without its limitations. Understanding them is key to interpreting your results correctly.
Qualitative Speed vs. Quantitative Rigor
A simple qualitative scan to identify a material can take mere seconds. Achieving high-accuracy quantitative results requires careful sample preparation, longer measurement times, and a rigorous calibration process that can take hours or even days to develop.
The "Matrix Effect" Challenge
The presence of other elements in the sample (the "matrix") can affect the X-ray signals. Heavy elements can absorb the signals from lighter ones, or secondary fluorescence can artificially boost other signals. Correcting for these matrix effects is a primary challenge in high-accuracy quantitative analysis.
A Surface-Level Technique
Standard XRF is a surface-sensitive technique. The X-rays typically only penetrate a few micrometers to a few millimeters into the material, depending on the sample's density. Therefore, the results only represent the composition of the surface, which may not be representative of the bulk material.
Light Element Detection
XRF struggles to detect very light elements (those with atomic numbers below ~11, like Sodium, Lithium, or Beryllium). Their characteristic X-rays are too low in energy and are often absorbed by the air or the detector window, making them difficult or impossible to measure with most standard XRF equipment.
Making the Right Choice for Your Goal
Your analytical objective determines whether you need a qualitative, semi-quantitative, or fully quantitative approach.
- If your primary focus is rapid material identification: A qualitative or semi-quantitative scan is sufficient for tasks like sorting scrap metal alloys, screening consumer products, or verifying material type.
- If your primary focus is precise compositional measurement: You must perform a full quantitative analysis with appropriate calibration standards for regulatory compliance (e.g., RoHS), quality control, or geochemical assays.
- If your primary focus is preliminary field screening: A semi-quantitative (standardless) analysis provides valuable estimates to guide decisions, such as identifying areas of interest in environmental soil testing before sending select samples for lab confirmation.
By understanding this dual nature, you can deploy XRF not just as a tool, but as a strategic analytical asset.
Summary Table:
| Analysis Type | Core Question | Key Requirement | Typical Output |
|---|---|---|---|
| Qualitative | "What elements are present?" | None (inherent) | Elemental spectrum/peaks |
| Quantitative | "How much of each element?" | Calibration with standards | Concentration (%, ppm) |
| Semi-Quantitative | "Approximately how much?" | Fundamental Parameters (FP) method | Estimated concentration |
Need precise elemental analysis for your laboratory? KINTEK specializes in high-performance XRF equipment and consumables, delivering the accuracy and reliability your lab demands. Whether you require rapid material identification or rigorous quantitative measurement, our solutions are tailored to meet your specific analytical goals. Contact our experts today to find the perfect XRF solution for your application!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Three-dimensional electromagnetic sieving instrument
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- How can corrosion of the sample holder be prevented when using corrosive chemicals? Protect Your Lab's Integrity
- Why is an airtight sample holder with a beryllium window required for XRD of sulfide solid electrolytes?
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions



















