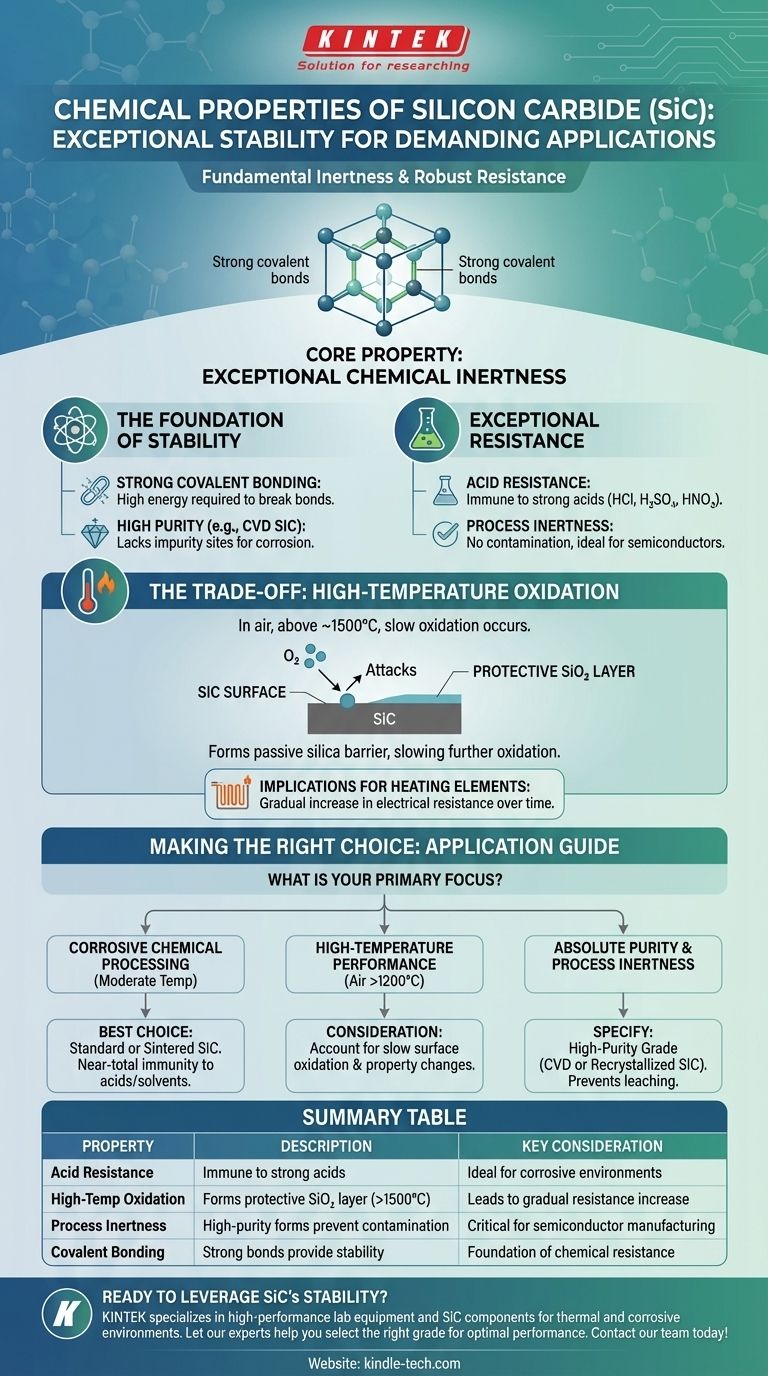
Fundamentally, silicon carbide (SiC) is defined by its exceptional chemical stability. It is a highly inert ceramic material, demonstrating remarkable resistance to chemical attack, particularly from strong acids. This inherent stability, combined with its other elite properties, is the primary reason it is specified for some of the most demanding industrial and high-technology applications.
Silicon carbide's core chemical property is its inertness. While it is virtually immune to acids and most chemicals at room temperature, its long-term stability becomes limited by slow oxidation when held at very high temperatures in the presence of air.
The Foundation of SiC's Chemical Stability
Silicon carbide's robust chemical nature is not an accident; it is a direct result of its atomic structure and the purity of its various manufactured forms.
Covalent Bonding and Purity
The powerful covalent bond between the silicon and carbon atoms requires a significant amount of energy to break, making the material inherently stable and non-reactive.
Furthermore, purer forms of SiC, such as CVD (Chemical Vapor Deposition) silicon carbide, exhibit a higher degree of chemical inertness because they lack the impurity sites where corrosive reactions could begin.
Exceptional Resistance to Acids
A key chemical property of SiC is its outstanding performance in corrosive environments.
It is extremely resistant to all strong acids, including hydrochloric, sulfuric, and nitric acids, and does not react with them. This makes it an ideal material for components used in chemical processing.
Inertness in Process Environments
This chemical stability also means SiC does not contaminate its surroundings.
Materials like CVD silicon carbide are considered to have a high degree of process inertness, which is critical in industries like semiconductor manufacturing where even trace amounts of contamination can ruin a product.
Understanding the Trade-off: High-Temperature Oxidation
While incredibly stable, silicon carbide is not infinitely inert. Its primary chemical vulnerability appears at very high temperatures in an oxidizing atmosphere.
The Oxidation Threshold
SiC can operate in air at temperatures up to approximately 1500°C. Beyond this range, and even during prolonged use within it, a slow reaction begins to occur.
The silicon within the SiC reacts with oxygen from the atmosphere. This is the material's most significant chemical limitation.
Formation of a Protective Layer
This reaction forms a thin, passive layer of silicon dioxide (SiO₂) on the surface.
This silica layer is glass-like and acts as a protective barrier, which dramatically slows the rate of any further oxidation and allows the component to continue functioning for a long service life.
Practical Implications for Heating Elements
This slow oxidation has tangible effects. For SiC heating elements, the formation of the SiO₂ layer causes the electrical resistance to gradually increase over time.
This change must be accounted for in the system design, often by using a variable power supply to maintain consistent heat output as the element ages.
Making the Right Choice for Your Goal
To properly apply silicon carbide, you must align its specific chemical properties with the demands of your environment.
- If your primary focus is chemical processing at moderate temperatures: SiC is an outstanding choice due to its near-total immunity to strong acids and chemical solvents.
- If your primary focus is high-temperature performance in air (above 1200°C): You must design around the reality of slow surface oxidation, which will gradually alter the material's surface properties.
- If your primary focus is absolute purity and process inertness: Specify a high-purity grade like CVD or recrystallized silicon carbide to prevent any potential for material leaching or contamination.
Understanding these nuances of silicon carbide's chemical behavior is the key to leveraging its remarkable stability in the correct context.

Summary Table:
| Key Chemical Property | Description | Key Consideration |
|---|---|---|
| Acid Resistance | Extremely resistant to all strong acids (HCl, H₂SO₄, HNO₃) | Ideal for corrosive chemical processing environments |
| High-Temperature Oxidation | Forms protective SiO₂ layer in air above ~1500°C | Leads to gradual resistance increase in heating elements |
| Process Inertness | High-purity forms (CVD) prevent contamination | Critical for semiconductor and high-purity manufacturing |
| Covalent Bonding | Strong atomic bonds provide inherent stability | Foundation of its robust chemical resistance |
Ready to leverage silicon carbide's exceptional chemical stability in your application? KINTEK specializes in high-performance lab equipment and consumables, including silicon carbide components for the most demanding thermal and corrosive environments. Our experts can help you select the right grade—from standard sintered to ultra-pure CVD SiC—to ensure optimal performance, longevity, and process purity. Contact our team today to discuss your specific needs and get a tailored solution!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Purity Zinc Foil for Battery Lab Applications
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Boron Nitride (BN) Ceramic Tube
People Also Ask
- What is a sputtering target used for? The Atomic Blueprint for High-Performance Thin Films
- What is the process of thin film optical coating? Precision Light Control for Lenses & Mirrors
- How is thin film coating done? A Guide to PVD, Sol-Gel, and Precision Deposition
- What is the principle of sputtering deposition? A Guide to High-Performance Thin Film Coating
- Can carbon nanotubes be mass produced? Scaling CNT Production for Commercial Applications
- What uses thin films? Discover the Invisible Tech Powering Modern Devices
- What is CVD lab grown diamond? A Real Diamond Grown in a Lab
- What is the sputtering process in nanotechnology? A Guide to Atomic-Level Thin Film Deposition