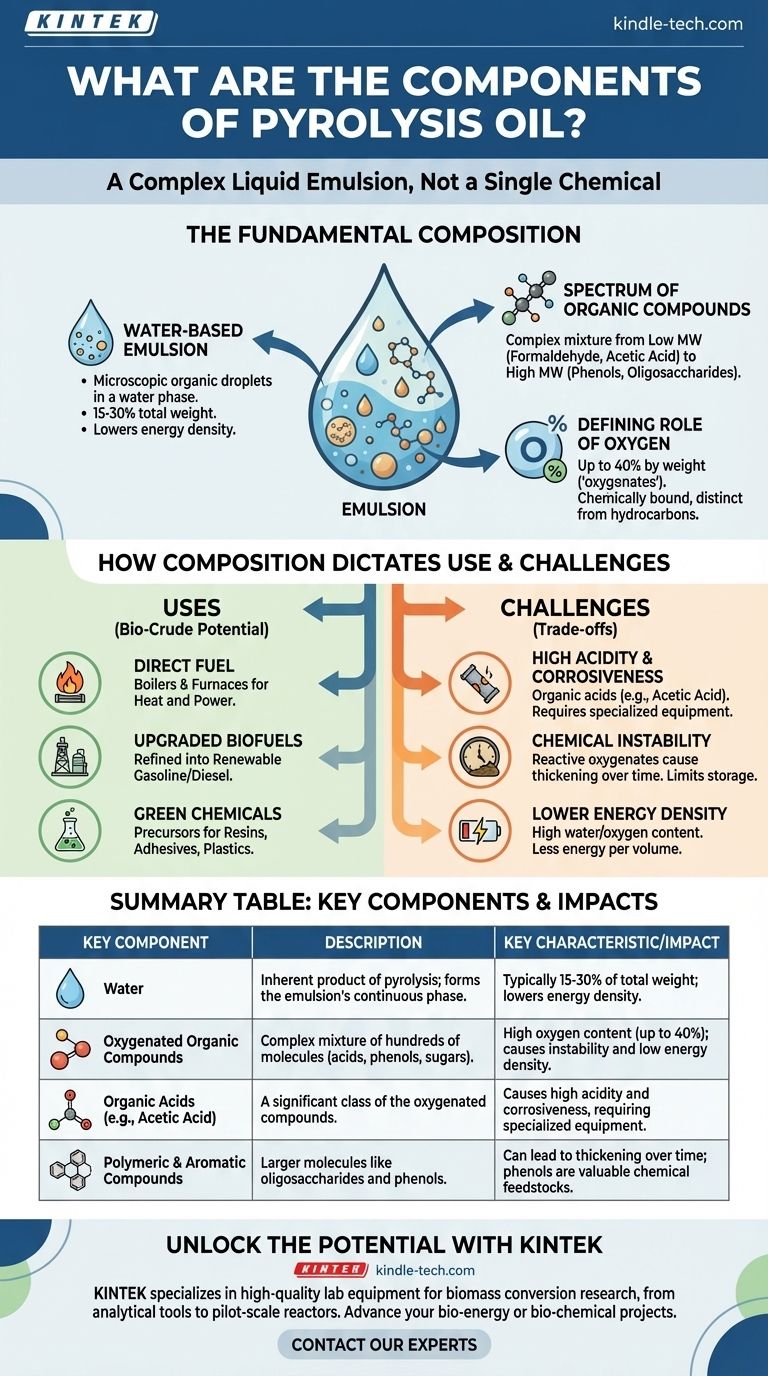
At its core, pyrolysis oil is not a single chemical but a complex liquid emulsion. It is primarily composed of hundreds of different oxygenated organic compounds, polymers, and a significant amount of water. This composition is a direct result of the rapid thermal decomposition of biomass in the absence of oxygen.
The key to understanding pyrolysis oil is recognizing its dual nature: it is a raw, acidic, and unstable mixture with high oxygen and water content, making it fundamentally different from conventional hydrocarbon fuels like diesel or crude oil.

The Fundamental Composition of Pyrolysis Oil
To grasp the nature of pyrolysis oil, often called "bio-crude," we must break down its primary components and their significance.
A Water-Based Emulsion
Pyrolysis oil is not a pure oil in the traditional sense. It is an emulsion, where microscopic droplets of organic compounds are suspended within a water phase. This water is not an impurity but an inherent product of the pyrolysis process.
A Spectrum of Organic Compounds
The organic portion is a vast and complex mixture of molecules with varying sizes and chemical functions.
These compounds range from very small, low molecular weight chemicals like formaldehyde and acetic acid to much larger, high molecular weight structures like phenols, other aromatic compounds, and oligosaccharides (chains of sugar molecules).
The Defining Role of Oxygen
The single most important characteristic of pyrolysis oil is its high oxygen content, which can be up to 40% by weight. This oxygen is chemically bound within the organic molecules, making them "oxygenates."
This is in stark contrast to conventional crude oil, which is almost entirely composed of hydrogen and carbon (hydrocarbons). This high oxygen content is directly responsible for many of the oil's unique properties.
How Composition Dictates Its Use and Challenges
The specific mix of water, acids, phenols, and other oxygenates determines how pyrolysis oil can be used and what difficulties it presents.
Use as a Direct Fuel
The organic compounds in the oil are combustible, making it a viable fuel source for stationary applications. It can be burned directly in industrial boilers and furnaces to generate heat and power.
A Feedstock for Upgraded Biofuels
While its properties make it unsuitable as a direct drop-in fuel for standard car engines, it is a critical intermediate. Through further refining processes (upgrading), the oxygen can be removed to produce more stable, energy-dense biofuels like renewable gasoline or diesel.
A Source for Green Chemicals
The complex mixture contains valuable chemical precursors. Phenols, for example, can be extracted and used as building blocks for creating products like resins, adhesives, and plastics, offering a renewable alternative to petroleum-derived chemicals.
Understanding the Trade-offs
The unique composition of pyrolysis oil creates significant challenges that must be managed. It is not a simple "plug-and-play" replacement for fossil fuels.
High Acidity and Corrosiveness
The presence of organic acids, most notably acetic acid, makes the oil highly acidic and corrosive to common metals like carbon steel. This requires specialized storage tanks, pumps, and transport equipment.
Chemical Instability
Pyrolysis oil is chemically unstable. The reactive oxygenated compounds can slowly react with each other over time, causing the oil to thicken, form sludge, and increase in viscosity. This limits its long-term storage potential.
Lower Energy Density
The high water and oxygen content mean that pyrolysis oil contains less energy per unit of volume compared to conventional hydrocarbon fuels. More of it must be burned to produce the same amount of heat.
Making the Right Choice for Your Goal
How you view pyrolysis oil depends entirely on your end objective.
- If your primary focus is simple heat generation: It can be used as a substitute for heavy fuel oil in industrial burners, provided the equipment can handle its corrosive nature.
- If your primary focus is producing transportation fuels: Treat it as a "bio-crude" that requires significant investment in upgrading and refining technology to become a finished product.
- If your primary focus is creating valuable bio-based chemicals: See it as a rich feedstock from which specific high-value chemical families can be extracted and purified.
Ultimately, understanding the complex composition of pyrolysis oil is the key to unlocking its potential as either a renewable fuel source or a sustainable chemical feedstock.
Summary Table:
| Key Component | Description | Key Characteristic/Impact |
|---|---|---|
| Water | Inherent product of pyrolysis; forms the emulsion's continuous phase. | Typically 15-30% of total weight; lowers energy density. |
| Oxygenated Organic Compounds | Complex mixture of hundreds of molecules (acids, phenols, sugars). | High oxygen content (up to 40%); causes instability and low energy density. |
| Organic Acids (e.g., Acetic Acid) | A significant class of the oxygenated compounds. | Causes high acidity and corrosiveness, requiring specialized equipment. |
| Polymeric & Aromatic Compounds | Larger molecules like oligosaccharides and phenols. | Can lead to thickening over time; phenols are valuable chemical feedstocks. |
Unlock the Potential of Bio-Based Feedstocks with KINTEK
Understanding the complex nature of pyrolysis oil is the first step toward leveraging it for renewable energy or green chemicals. Whether your goal is to develop efficient pyrolysis processes, upgrade bio-oil into advanced biofuels, or extract valuable chemical precursors, having the right laboratory equipment is critical.
KINTEK specializes in supplying high-quality lab equipment and consumables for biomass conversion research and development. From analytical tools to pilot-scale reactors, we provide the reliable technology you need to analyze, process, and innovate with pyrolysis oil and other bio-based materials.
Ready to advance your bio-energy or bio-chemical projects? Contact our experts today to discuss how KINTEK's solutions can support your specific laboratory needs and help you achieve your sustainability goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Lab-Scale Vacuum Induction Melting Furnace
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- How do laboratory shakers or stirrers influence the efficiency of hydrogen production during dark fermentation?
- Is sintering the same as melting? Master the Critical Thermal Process Distinction
- What is sintering with an example? A Guide to Fusing Powders into Solid Parts
- What is the primary function of a laboratory oven in biomass treatment? Ensure Accurate Dry-Basis Analysis
- What are the alternative pressing and sintering techniques? Overcome the Limits of Conventional Powder Metallurgy
- What temperature should brazing be? Master the Key to Strong, Reliable Joints
- What is a gold sputtering target? A High-Purity Source for Precision Gold Coatings
- Can pyrolysis produce electricity? Unlock the Potential of Waste-to-Energy Systems





