In microbiology, sterilization is achieved through several primary methods, each based on different physical or chemical principles. The most common and reliable method is steam sterilization using an autoclave, which employs saturated steam under high pressure and temperature. Other principal methods include dry heat, chemical sterilization, radiation, and filtration, chosen based on the material being sterilized and the specific laboratory requirement.
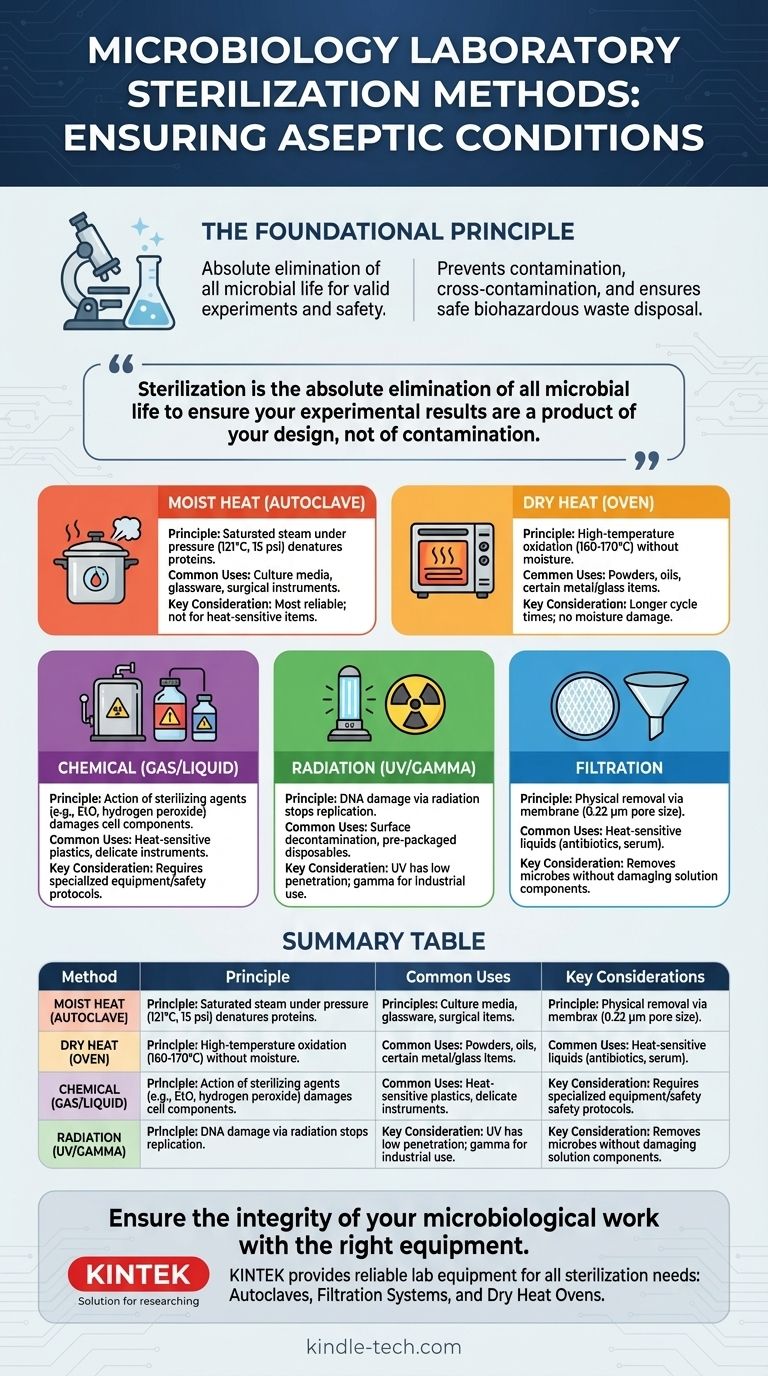
The core principle of laboratory work is control. Sterilization is not simply about cleaning; it is the absolute elimination of all microbial life to ensure that your experimental results are a product of your design, not of contamination.

The Foundational Principle: Why Sterilization is Non-Negotiable
Effective sterilization is the bedrock of microbiology. Without it, every experiment would be invalid, and the handling of biological materials would be unacceptably hazardous.
Ensuring Aseptic Conditions
Aseptic technique refers to the practice of preventing contamination. Sterilization ensures that your starting materials—media, glassware, and instruments—are a "blank slate," completely free of any bacteria, fungi, or viruses that could interfere with your work.
Preventing Cross-Contamination
When working with multiple microbial cultures, even a single stray cell can be transferred from one sample to another via a contaminated instrument. Sterilizing tools like inoculation loops and glassware between uses is critical for maintaining the purity of each culture.
Safe Disposal of Biohazardous Waste
After an experiment, all used materials, such as petri dishes and culture tubes, are considered biohazardous waste. Sterilizing this waste, typically in an autoclave, is a mandatory safety protocol to kill the microorganisms before the materials are discarded, protecting both lab personnel and the environment.
Primary Sterilization Methods: A Detailed Breakdown
The choice of method depends entirely on the nature of the item you need to sterilize. Heat is the most common approach, but it is not universally applicable.
Heat Sterilization: The Workhorse of the Lab
Heat kills microbes by denaturing their essential proteins and enzymes. There are two primary forms of heat sterilization.
-
Moist Heat (Autoclaving): This is the gold standard. An autoclave is essentially a highly sophisticated pressure cooker. It uses steam heated to 121°C (250°F) at a pressure of 15 psi for at least 15-20 minutes. The high pressure allows the steam to reach temperatures above the boiling point of water, and the moisture rapidly transfers heat, efficiently killing even resilient bacterial spores. It is used for culture media, glassware, and surgical instruments.
-
Dry Heat (Hot Air Oven): This method uses high temperatures without moisture, typically 160-170°C (320-340°F) for 2-3 hours. It kills microbes through oxidation. Dry heat is less efficient than moist heat but is necessary for materials that cannot be exposed to steam, such as oils, powders, and certain metal or glass items.
Chemical Sterilization: For Heat-Sensitive Items
Many essential lab items, especially plastics and complex electronics, cannot withstand the high temperatures of an autoclave. For these, chemical agents are used.
- Gaseous Methods: Gases like Ethylene Oxide (EtO) are used to sterilize heat-sensitive items like plastic petri dishes, syringes, and catheters on an industrial scale.
- Liquid Methods: Highly reactive chemicals like hydrogen peroxide or glutaraldehyde can be used as high-level disinfectants or chemical sterilants for delicate instruments.
Radiation Sterilization: High-Energy Penetration
Radiation damages the DNA of microorganisms, rendering them unable to replicate.
- Ionizing Radiation (Gamma, X-ray): This high-energy radiation has deep penetrating power and is used for the industrial sterilization of pre-packaged disposables like gloves, syringes, and swabs. You typically receive these items already sterilized by this method.
- Non-ionizing Radiation (UV Light): Ultraviolet radiation has poor penetrating power and is primarily used for surface decontamination. It is commonly found in biological safety cabinets to sterilize the air and work surfaces.
Filtration: Removing Microbes from Liquids
Sometimes you need to sterilize a liquid that contains heat-sensitive components, such as vitamins, antibiotics, or certain proteins. In this case, killing the microbes with heat would also destroy the valuable components.
Filtration works by passing the liquid through a membrane with pores so small (typically 0.22 micrometers) that bacteria cannot pass through. This physically removes the microbes rather than killing them, leaving the heat-sensitive solution sterile.
Understanding the Trade-offs
No single method is perfect for every situation. Understanding their limitations is key to making the correct choice.
Efficacy vs. Material Compatibility
The autoclave is the most effective and reliable method, but its high heat and moisture will melt many plastics and damage sensitive electronics. Dry heat is an alternative for some items but can be corrosive over time and is unsuitable for liquids.
Penetration Power
Moist heat and ionizing radiation have excellent penetration, ensuring items are sterilized inside and out. In contrast, UV light only sterilizes the direct surface it shines upon, leaving "shadowed" areas contaminated.
Safety and Accessibility
Autoclaves operate under high pressure and temperature and must be used with proper training to avoid serious injury. Chemical sterilants like EtO are highly toxic and require specialized, ventilated chambers. Filtration and dry heat ovens are generally safer and more accessible for routine lab work.
Making the Right Choice for Your Goal
Your objective dictates your method. Choose the most effective and efficient option that will not damage your materials.
- If your primary focus is sterilizing most culture media, glassware, and metal tools: The autoclave is your definitive choice for its unmatched reliability and efficiency.
- If your primary focus is sterilizing heat-sensitive liquids like antibiotic solutions or cell culture media: Membrane filtration is the only method that preserves the integrity of your solution.
- If your primary focus is sterilizing dry powders, oils, or certain metal instruments: A dry heat oven is the correct tool, as moist steam would be ineffective or damaging.
- If your primary focus is decontaminating a work surface or the air inside a biosafety cabinet: UV radiation is the standard and appropriate method for surface-level decontamination.
Selecting the correct sterilization method is the foundation of reliable and safe microbiological practice.
Summary Table:
| Method | Principle | Common Uses | Key Considerations |
|---|---|---|---|
| Moist Heat (Autoclave) | Saturated steam under pressure (121°C) | Culture media, glassware, surgical instruments | Most reliable; not for heat-sensitive plastics/electronics |
| Dry Heat (Oven) | High-temperature oxidation (160-170°C) | Powders, oils, certain metal/glass items | Longer cycle times; no moisture damage |
| Chemical (Gas/Liquid) | Action of sterilizing agents (e.g., EtO) | Heat-sensitive plastics, delicate instruments | Often requires specialized equipment/safety protocols |
| Radiation (UV/Gamma) | DNA damage via radiation | Surface decontamination, pre-packaged disposables | UV has low penetration; gamma for industrial use |
| Filtration | Physical removal via membrane (0.22 µm) | Heat-sensitive liquids (antibiotics, serum) | Removes microbes without damaging solution components |
Ensure the integrity of your microbiological work with the right equipment.
KINTEK specializes in providing reliable lab equipment and consumables for all your sterilization needs. Whether you require robust autoclaves for media and glassware, precise filtration systems for sensitive solutions, or safe dry heat ovens, we have the solutions to support your laboratory's safety and efficiency.
Contact us today to find the perfect sterilization equipment for your specific applications and ensure contaminant-free results.
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
People Also Ask
- What precautions should be taken during autoclave in laboratory? A Complete Safety Guide to Prevent Burns and Explosions
- What is the function of high-pressure autoclaves in IASCC testing? Ensure Nuclear Material Safety
- What is the function of an industrial-grade 316 stainless steel autoclave? Master PWR Secondary Circuit Simulation
- What is the research value of using a high-pressure autoclave for curing geopolymer materials? Unlocking Durability
- What is the size of an autoclave sterilizer? Find the Perfect Fit for Your Lab's Needs
- Why is a laboratory autoclave necessary for carbohydrate composition analysis? Unlock Accurate Sugar Yields
- When should an autoclave not be used? Avoid Damage and Hazards in Your Lab
- What is the role of a PTFE-lined stainless steel autoclave? Master Gamma-AlOOH Synthesis with High Purity



















