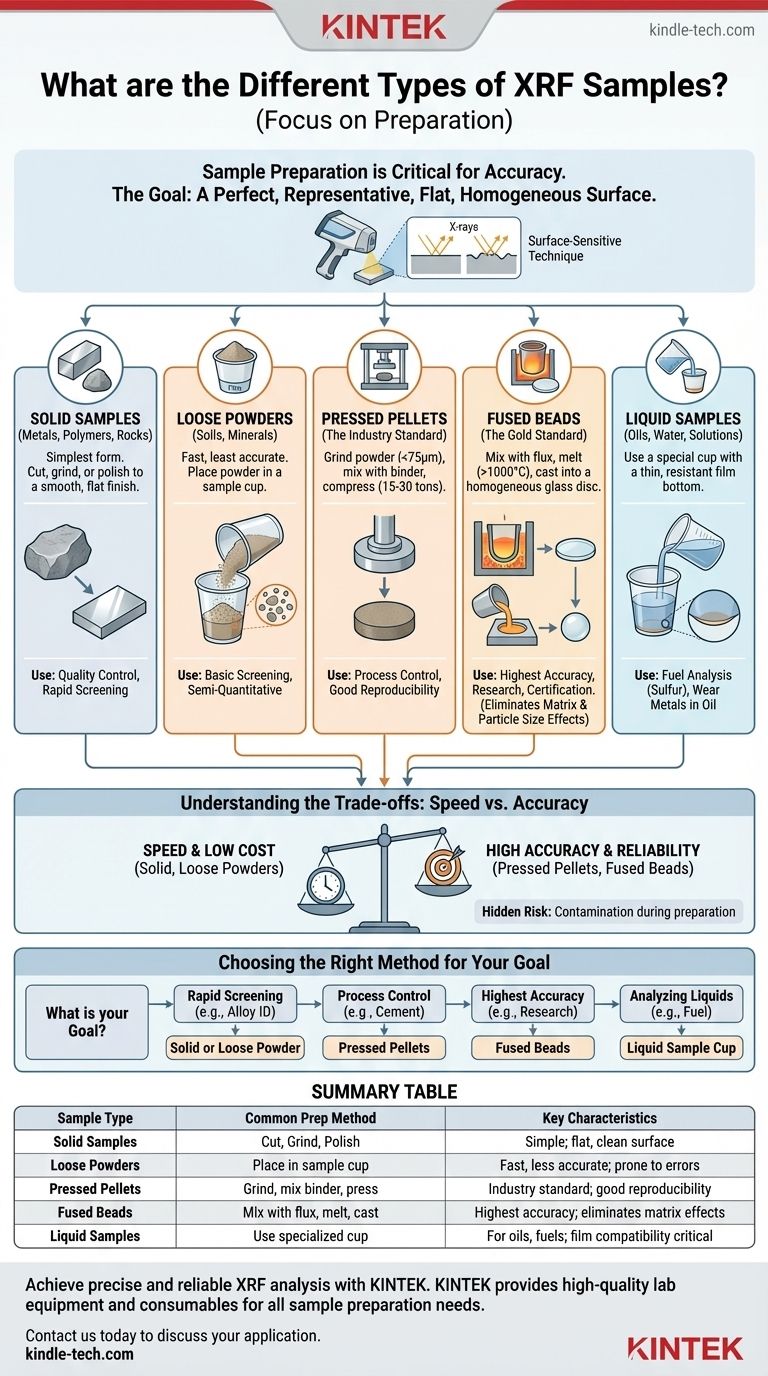
For X-ray Fluorescence (XRF) analysis, samples are typically classified into three main physical forms: solids, powders, and liquids. However, the most critical distinction is not the sample's initial state, but the preparation method used to create a flat, homogeneous surface for analysis. These methods range from analyzing a solid "as-is" to pressing powders into pellets or melting them into perfect glass discs known as fused beads.
The accuracy of your XRF results is determined almost entirely by your sample preparation. The core objective is always the same: to present a perfectly flat, dense, and chemically uniform surface to the spectrometer, thereby minimizing physical and chemical errors that can distort the measurement.

Why Sample Preparation is Critical
XRF is a surface-sensitive technique. The primary X-rays only penetrate a few micrometers to millimeters into the sample, meaning the spectrometer only "sees" the very top layer. If this layer isn't a perfect representation of the entire sample, the results will be inaccurate.
The Goal: A Perfect, Representative Surface
The ideal sample is infinitely thick from the instrument's perspective, perfectly flat, and completely homogeneous. Any deviation from this ideal introduces errors. Proper preparation aims to transform a non-ideal, real-world sample into one that closely mimics this perfect state.
Eliminating Physical Errors
The physical nature of a sample can dramatically alter X-ray signals.
- Particle Size Effects: In powders, larger particles can shadow smaller ones, and different mineral phases can absorb or enhance fluorescence differently. Grinding the sample to a very fine, uniform particle size is essential.
- Surface Roughness: An uneven surface on a solid sample will scatter X-rays unpredictably, leading to inconsistent and inaccurate results. The surface must be flat and smooth.
- Voids and Density: Gaps between particles in a loose powder or pressed pellet reduce the sample's density in the analysis area, lowering the measured intensity for all elements.
Mitigating Chemical "Matrix Effects"
The collection of all atoms in a sample is called the matrix. The presence of certain elements can absorb or enhance the fluorescent X-rays from the element you are trying to measure. Fusing the sample into a glass bead is the most effective way to drastically reduce these chemical interferences.
Common XRF Sample Types and Preparation Methods
The method you choose depends on the sample material, the required accuracy, and the available equipment.
Solid Samples (Metals, Polymers, Rocks)
This is the simplest form of preparation, often used for quality control in manufacturing. The sample must be large enough to cover the analysis area and flat enough to sit flush in the spectrometer.
Preparation involves cutting a representative piece and then preparing the surface by lathing, grinding, or polishing to remove any contamination and create a smooth, flat finish.
Loose Powders (Soils, Minerals)
This is the fastest but least accurate method for powdered materials. The powder is simply placed into a sample cup that has a thin, X-ray transparent film for a base.
This method is highly susceptible to errors from inconsistent density and particle size effects. It is only suitable for basic screening or when high accuracy is not the primary concern.
Pressed Pellets (The Industry Standard)
This is the most common method for preparing powdered samples like minerals, cement, and soils. The sample is first ground into a fine powder (<75 microns), often mixed with a binder, and then compressed under high pressure (15-30 tons) to form a dense, durable pellet.
This technique vastly improves on loose powders by creating a sample with a uniform density and a flat surface, leading to much higher precision and reproducibility.
Fused Beads (The Gold Standard)
For applications demanding the highest accuracy, fusion is the ultimate method. The powdered sample is mixed with a lithium borate flux, heated in a platinum crucible to over 1000°C until it melts, and then cast into a mold to cool as a perfectly homogeneous glass disc.
This process destroys the original crystal structure, eliminating all particle size and mineralogical effects. It also dilutes the sample, which significantly reduces chemical matrix effects, yielding the most accurate results possible.
Liquid Samples (Oils, Water, Solutions)
Liquids are analyzed using a special cup with a thin film bottom, similar to those for loose powders. Care must be taken to ensure the film is chemically resistant to the liquid and that no leaks occur.
This method is standard for analyzing elements like sulfur in fuel or monitoring wear metals in lubricating oils.
Understanding the Trade-offs: Speed vs. Accuracy
No single method is perfect for every situation. You must balance the need for quality results with practical limitations like time and cost.
The "Minimal Prep" Approach
Analyzing solid samples "as-is" or using loose powders is fast and requires little equipment. This is ideal for rapid screening or when only semi-quantitative data is needed. The trade-off is significantly lower accuracy and precision.
The "High-Effort" Approach
Pressed pellets and fused beads require specialized equipment (mills, presses, fusion machines) and take more time and skill. Fusion is also a destructive technique. However, these methods deliver the superior accuracy, precision, and reliability required for process control, research, and certification.
The Hidden Risk: Contamination
Throughout any preparation process, contamination is a constant risk. Material from grinding equipment, impurities in binders or fluxes, or simple mishandling can introduce elements into the sample, leading to false readings.
Choosing the Right Method for Your Goal
Your analytical goal should dictate your sample preparation strategy.
- If your primary focus is rapid sorting or screening (e.g., alloy identification, basic QC): Analyzing a clean, flat solid sample or a loose powder is often sufficient.
- If your primary focus is process control with good reproducibility (e.g., cement manufacturing, mining): Pressed pellets offer the best balance of speed, cost, and precision.
- If your primary focus is the highest possible accuracy for research or certification (e.g., geological surveys, creating reference materials): Fused beads are the undisputed best method, as they eliminate most sources of error.
- If your primary focus is analyzing liquids (e.g., sulfur in fuel, wear metals in oil): Use a dedicated liquid sample cup, ensuring the support film is compatible with your material.
Ultimately, selecting and perfecting the correct sample preparation technique is the single most important step in achieving reliable and trustworthy XRF results.
Summary Table:
| Sample Type | Common Preparation Method | Key Characteristics |
|---|---|---|
| Solid Samples | Cut, Grind, Polish | Simple; requires flat, clean surface |
| Loose Powders | Place in sample cup | Fast but less accurate; prone to errors |
| Pressed Pellets | Grind, mix with binder, press | Industry standard; good reproducibility |
| Fused Beads | Mix with flux, melt, cast | Highest accuracy; eliminates matrix effects |
| Liquid Samples | Use specialized sample cup | For oils, fuels, solutions; requires film compatibility |
Achieve precise and reliable XRF analysis with KINTEK.
Choosing the right sample preparation method is critical for accurate results. Whether you need the speed of pressed pellets for process control or the ultimate accuracy of fused beads for research, KINTEK provides the high-quality lab equipment and consumables to meet your needs. Our expertise in XRF sample preparation ensures you minimize errors and maximize data reliability.
Contact us today to discuss your specific application and let our specialists help you select the ideal solution. Get in touch via our contact form to enhance your laboratory's capabilities!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
People Also Ask
- Why is a hydraulic pellet press used for FTIR? Transform Nanofillers into Clear Data
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- What is the function of a bench-top laboratory hydraulic press for XRF? Maximize Accuracy in Prosopis juliflora Analysis
- What is the use of manual hydraulic press? A Cost-Effective Tool for Lab Sample Preparation
- What is KBr disc method? A Complete Guide to IR Spectroscopy Sample Prep



















