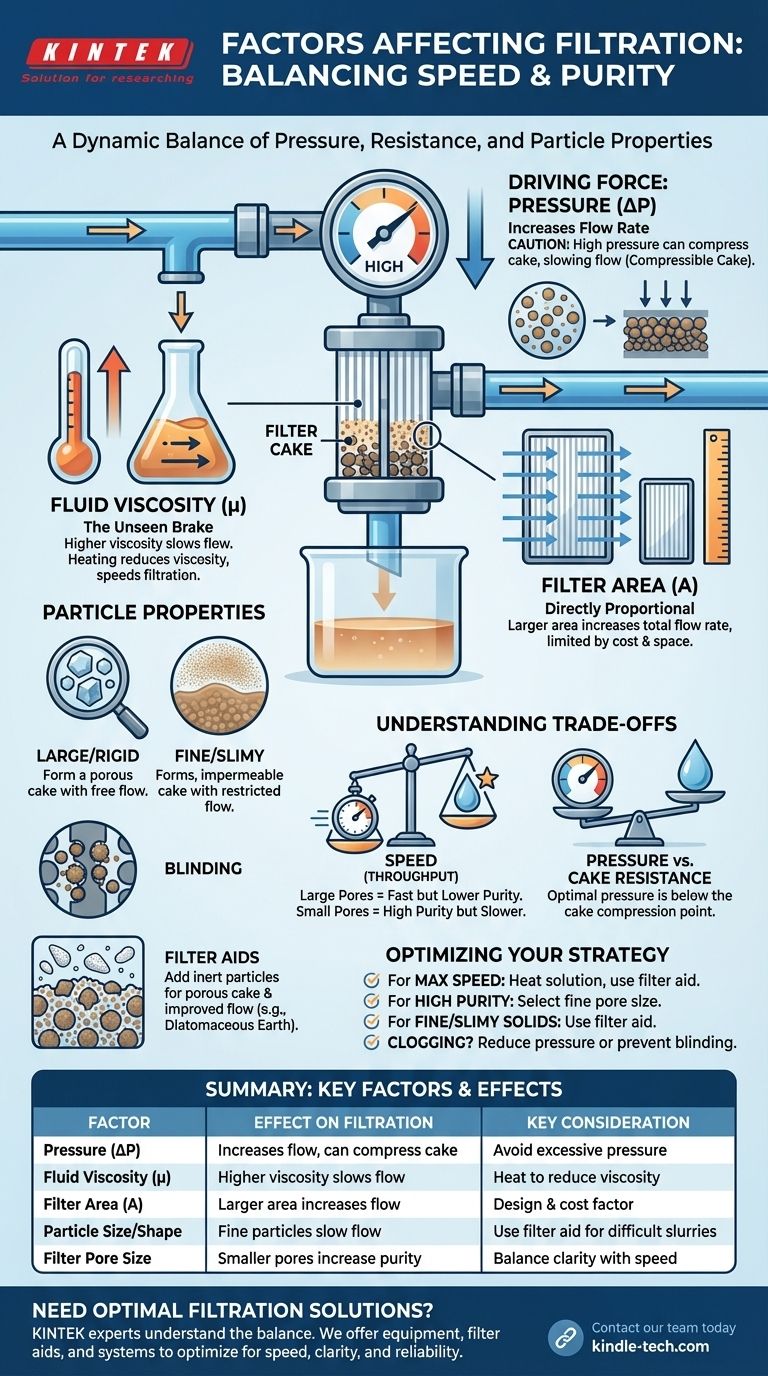
In any filtration process, the rate and efficiency are governed by a core set of physical factors. The most critical of these are the pressure difference across the filter, the viscosity of the fluid, the surface area of the filter, and the nature of the solid particles being removed, which form a resistive "cake" on the filter medium.
The central challenge of filtration is not merely pushing a liquid through a screen. It is a dynamic balance between the driving force (pressure) and the constantly increasing resistance from both the filter medium and the accumulating solids. Mastering filtration requires managing this resistance.

The Driving Force: Pressure Differential (ΔP)
The fundamental driver of filtration is the pressure applied to the solution. This force pushes the liquid phase (filtrate) through the pores of the filter medium, leaving the solid phase behind.
How Pressure Increases Flow Rate
All else being equal, a higher pressure differential (the difference in pressure before and after the filter) results in a faster flow rate. This relationship is linear at first, as described by Darcy's Law, which models flow through a porous medium.
Doubling the effective pressure will, in an ideal scenario, double the speed of filtration.
The Limit of Pressure: Cake Compressibility
However, simply increasing pressure is not always the answer. Many solids form a "compressible cake," meaning the particles deform and pack closer together under high pressure.
This compression reduces the empty space within the cake, drastically increasing its resistance to flow. Beyond a certain point, adding more pressure can actually slow down or even stop filtration by creating an impassable barrier.
The Inherent Resistance: Fluid and Filter Properties
Before the solids even begin to build up, the system has a baseline resistance determined by the fluid itself and the chosen filter.
Fluid Viscosity (μ): The Unseen Brake
Viscosity is a measure of a fluid's resistance to flow—think of the difference between filtering water and filtering honey. A higher viscosity fluid will always filter more slowly.
This factor is inversely proportional to the flow rate. If you can cut the fluid's viscosity in half, you will double the filtration speed, assuming all other factors remain constant.
Temperature's Role in Reducing Viscosity
For most liquids, viscosity decreases significantly as temperature increases. Heating your solution (if the product is stable at higher temperatures) is one of the most effective ways to speed up a slow filtration process by lowering the fluid's viscosity.
Filter Area (A): A Matter of Scale
The total available surface area of the filter is directly proportional to the overall flow rate. Doubling the filter area will double the amount of filtrate you can collect in a given time.
This is primarily an equipment design choice, often limited by cost and physical space.
The Filter Medium: Pore Size and Material
The filter medium itself presents a fixed resistance. The key property is its pore size.
Smaller pores provide a clearer filtrate by capturing finer particles, but they also create more resistance and are more susceptible to clogging, resulting in a slower process.
The Evolving Challenge: The Filter Cake
The most complex factor in filtration is the layer of solids that accumulates on the surface of the filter medium. This "filter cake" often presents far more resistance than the filter medium itself.
Particle Size and Shape
Large, crystalline, and incompressible particles form a porous cake with plenty of channels for liquid to flow through. This results in fast and efficient filtration.
Conversely, very fine, amorphous, or "slimy" particles tend to form a dense, compressible, and low-permeability cake that severely impedes flow.
Solid Concentration (Slurry Density)
A solution with a high concentration of solids will form a thick filter cake much faster than a dilute solution. This increases resistance rapidly and shortens the effective time of each filtration cycle.
The Problem of "Blinding"
Blinding occurs when particles become lodged within the pores of the filter medium itself, rather than just sitting on the surface. This is different from simple surface clogging and can permanently reduce the filter's effectiveness, often requiring aggressive cleaning or replacement.
Understanding the Trade-offs
Optimizing filtration always involves balancing competing factors. There is no single "best" method, only the best method for a specific goal.
Speed vs. Purity (Pore Size Dilemma)
Using a filter with large pores will yield a fast filtration rate but may allow fine particles to pass through into the filtrate. A filter with very fine pores will produce a highly pure filtrate but at the cost of a much slower process.
Pressure vs. Cake Resistance
Applying high pressure can overcome initial resistance, but for compressible solids, it will eventually work against you by compacting the filter cake into an impermeable slab. The optimal pressure is often just below the point where cake compression becomes a significant factor.
The Role of Filter Aids
For difficult-to-filter slurries with fine or gelatinous solids, a filter aid (such as diatomaceous earth or perlite) can be added. These inert, incompressible particles mix with the solids to create a porous, stable filter cake, dramatically improving flow rate and preventing blinding.
Optimizing Your Filtration Process
Your strategy should be dictated by your primary objective. By understanding the principles above, you can make targeted adjustments.
- If your primary focus is maximizing throughput (speed): Consider gently heating the solution to reduce viscosity and using a filter aid to maintain cake permeability.
- If your primary focus is achieving high purity (clarity): Choose a filter medium with the appropriate fine pore size and accept that the process will be slower.
- If you are dealing with fine or "slimy" solids: Using a filter aid is almost always the best strategy to create a permeable cake structure.
- If your process is clogging prematurely: You may be using too much pressure, causing cake compression, or your particles may be blinding the filter medium, requiring a pre-treatment step.
By understanding these interconnected factors, you can move from troubleshooting filtration to strategically designing it for optimal performance.
Summary Table:
| Factor | Effect on Filtration | Key Consideration |
|---|---|---|
| Pressure (ΔP) | Increases flow rate | Can compress cake and slow flow if too high |
| Fluid Viscosity (μ) | Higher viscosity slows flow | Heating reduces viscosity and speeds filtration |
| Filter Area (A) | Larger area increases flow rate | An equipment design and cost consideration |
| Particle Size/Shape | Large, rigid particles flow fast; fine, slimy particles slow flow | Use a filter aid for difficult slurries |
| Filter Pore Size | Smaller pores increase purity but decrease speed | Balance clarity requirements with throughput needs |
Struggling with slow or inefficient filtration in your lab? The experts at KINTEK understand the delicate balance of pressure, viscosity, and particle properties. We specialize in providing the right lab equipment and consumables—from robust filtration systems to effective filter aids—to optimize your process for speed, clarity, and reliability.
Let us help you design a better filtration strategy. Contact our team today to discuss your specific application and challenges.
Visual Guide
Related Products
- Hydraulic Diaphragm Lab Filter Press for Laboratory Filtration
- Narrow Band Pass Filters for Precision Applications
- Longpass Highpass Filters for Optical Applications
- Three-dimensional electromagnetic sieving instrument
- Vibratory Sieve Shaker Machine Dry Three-Dimensional Vibrating Sieve
People Also Ask
- What are some of the problems related to hydraulic power? Manage Leaks, Contamination, and Inefficiency
- What are the preventive maintenance of hydraulic systems? Extend Equipment Life and Maximize Uptime
- What happens if a hydraulic system leaks? Prevent Costly Damage and Safety Hazards
- What are the most common causes of hydraulic system failure? Prevent Downtime and Costly Repairs
- What is the lifespan of a filter media? Understand the 3 Types for Optimal Filtration








