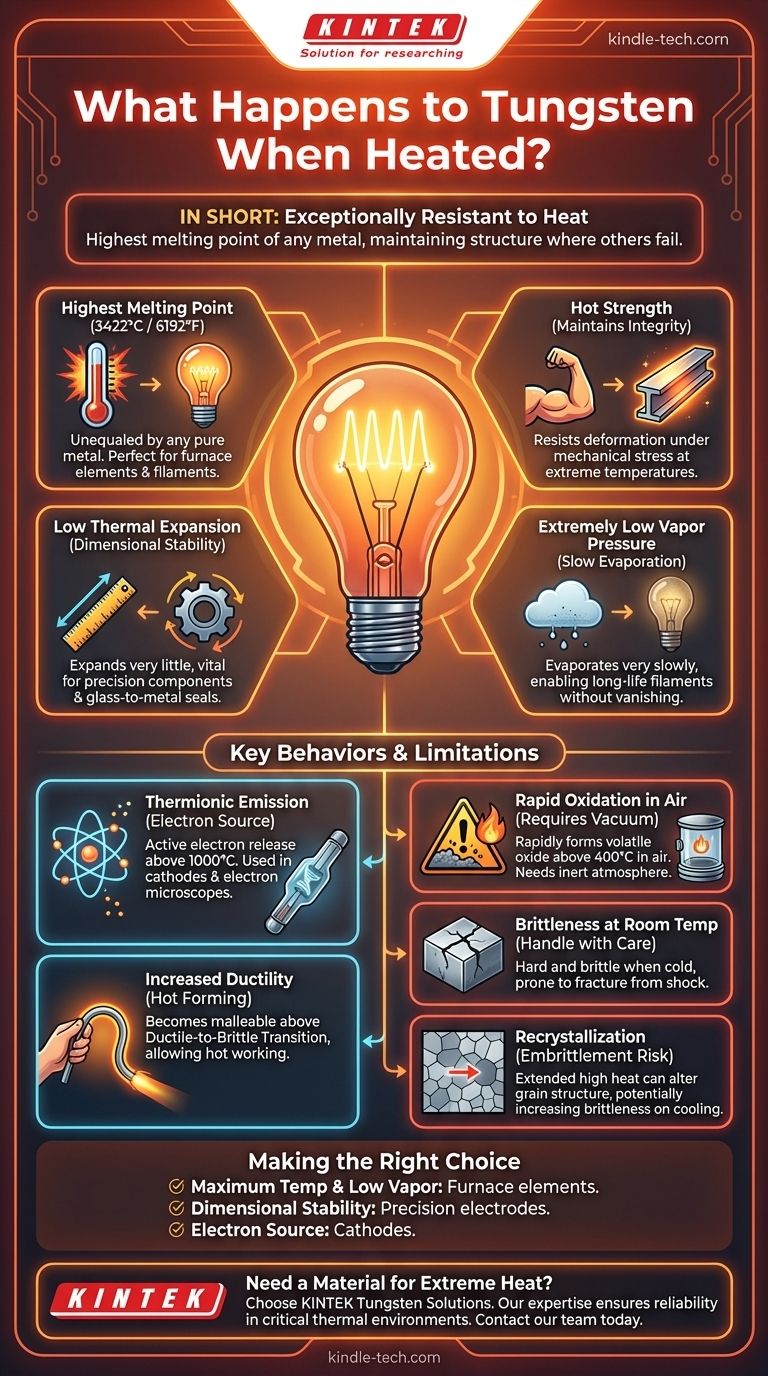
In short, tungsten is exceptionally resistant to heat. Unlike most materials, it remains solid and structurally stable at incredibly high temperatures, boasting the highest melting point of any metal. This unique property, combined with its strength and low rate of expansion, is precisely why it is chosen for some of the most demanding high-temperature applications.
The crucial insight is that tungsten's value comes from a combination of properties that manifest under extreme heat: not only its high melting point, but also its low vapor pressure and its ability to maintain strength, which together prevent it from melting, evaporating, or deforming where other metals would instantly fail.
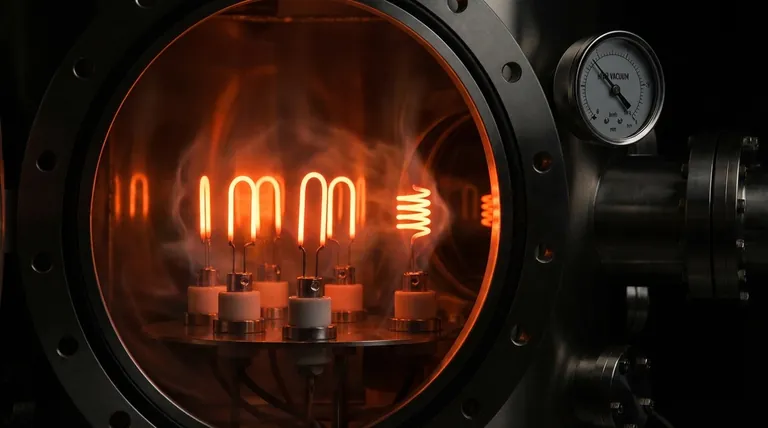
The Defining Characteristic: An Extremely High Melting Point
The Highest of All Pure Metals
The most famous property of tungsten is its melting point of 3422°C (6192°F). This is higher than any other pure metal on the periodic table.
This fundamental characteristic is the primary reason tungsten is the material of choice for applications like incandescent bulb filaments and heating elements in high-temperature vacuum furnaces.
Maintaining Structural Integrity ("Hot Strength")
Tungsten doesn't just resist melting; it also stays remarkably strong and hard at temperatures that would turn steel and even titanium soft.
This property, known as hot strength or hot hardness, ensures that components made from tungsten maintain their shape and integrity under mechanical stress, even when glowing white-hot.
Key Physical Behaviors Under Heat
Beyond simply not melting, tungsten exhibits several other critical behaviors when heated that define its use in engineering.
Low Thermal Expansion
Compared to most metals, tungsten expands very little when heated. This dimensional stability is vital for precision components that must maintain their exact shape and tolerances through extreme temperature cycles.
Its coefficient of thermal expansion is similar to that of borosilicate glass, making it an excellent material for creating airtight glass-to-metal seals in vacuum tubes and lamps.
Extremely Low Vapor Pressure
Even well below its melting point, a heated material can lose mass through sublimation or evaporation. Tungsten has an extremely low vapor pressure, meaning it evaporates very slowly even at thousands of degrees.
This is what allows a light bulb filament to glow for over a thousand hours without simply vanishing into the inert gas that fills the bulb.
Thermionic Emission
At very high temperatures (typically above 1000°C), tungsten begins to actively "boil off" electrons from its surface.
This phenomenon, called thermionic emission, is harnessed in applications where a reliable source of electrons is needed, such as in X-ray tubes, electron microscopes, and cathode-ray tubes.
Increased Ductility
While famously brittle at room temperature, tungsten becomes more ductile and easier to form and shape when heated above its Ductile-to-Brittle Transition Temperature (DBTT).
This property is exploited during manufacturing, where tungsten is often drawn into wires or worked into complex shapes while hot.
Understanding the Trade-offs and Limitations
Tungsten's remarkable high-temperature performance comes with critical limitations that must be managed.
Rapid Oxidation in Air
Tungsten's most significant weakness is its poor resistance to oxidation. When heated in the presence of oxygen (air) above approximately 400°C (750°F), it begins to rapidly form a volatile tungsten oxide.
This is why high-temperature tungsten applications must operate in a vacuum or a protective, inert gas atmosphere like argon or nitrogen. An incandescent filament would burn out in seconds if exposed to air.
Brittleness at Room Temperature
The same atomic structure that gives tungsten its strength also makes it very brittle and difficult to machine when cold. It is prone to fracturing from shock or impact.
Engineers must carefully design around this brittleness, avoiding sharp corners and impact loads in components operating at lower temperatures.
Recrystallization and Embrittlement
Holding tungsten at very high temperatures for extended periods can cause its internal grain structure to change, a process called recrystallization.
While this can make it softer while hot, it can also lead to increased brittleness once the material cools back down, potentially reducing the service life of a component.
Making the Right Choice for Your Application
- If your primary focus is maximum temperature resistance in a vacuum: Tungsten is the premier choice for applications like furnace elements and evaporation coils due to its unmatched melting point and low vapor pressure.
- If your primary focus is dimensional stability during thermal cycling: Its low coefficient of thermal expansion makes it ideal for precision components like electrodes and glass-to-metal seals.
- If your primary focus is creating an electron source: Tungsten's ability to perform thermionic emission makes it essential for cathodes in devices like X-ray tubes and electron microscopes.
- If your application operates in an oxygen-rich atmosphere above 400°C: You must either use a protective coating, alloy the tungsten, or choose a different class of material entirely, as pure tungsten will rapidly fail.
Ultimately, tungsten's behavior under heat makes it an extraordinary material for creating reliability in the most extreme thermal environments imaginable.
Summary Table:
| Property | Behavior Under Heat | Key Application |
|---|---|---|
| Melting Point | Highest of all pure metals (3422°C) | Furnace heating elements |
| Hot Strength | Maintains strength at white-hot temperatures | Structural components under stress |
| Thermal Expansion | Very low (dimensional stability) | Glass-to-metal seals, precision electrodes |
| Vapor Pressure | Extremely low (slow evaporation) | Incandescent lamp filaments |
| Oxidation | Rapid above 400°C in air | Requires vacuum/inert atmosphere |
Need a Material for Extreme Heat? Choose KINTEK Tungsten Solutions.
Tungsten's unique combination of properties—highest melting point, exceptional hot strength, and dimensional stability—makes it indispensable for demanding high-temperature applications. Whether you're designing furnace components, precision electrodes, or specialized cathodes, KINTEK's expertise in tungsten lab equipment and consumables ensures reliability in your most critical thermal environments.
Let our specialists help you select the right tungsten-based solution for your specific high-temperature needs. Contact our team today to discuss how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Tungsten Evaporation Boat for Thin Film Deposition
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
People Also Ask
- What is molybdenum highest melting point? 2622°C for Extreme Heat Applications
- Do heating elements degrade over time? Understanding the Inevitable Decay for Better Performance
- Is tungsten the most heat resistant material? It depends on your application's environment.
- What are the typical industrial applications for PTC heating elements? Explore Efficient Point Heating Solutions
- Why is the resistance of a heating element high? To Efficiently Convert Electricity into Heat
- What are the technical advantages of using graphite rods? Boost Precision in 1200°C High-Temperature Operations
- How does a heating element heat up? The Science of Joule Heating Explained
- How do you control the temperature of a heating element? Master On/Off, Proportional, and PID Methods












