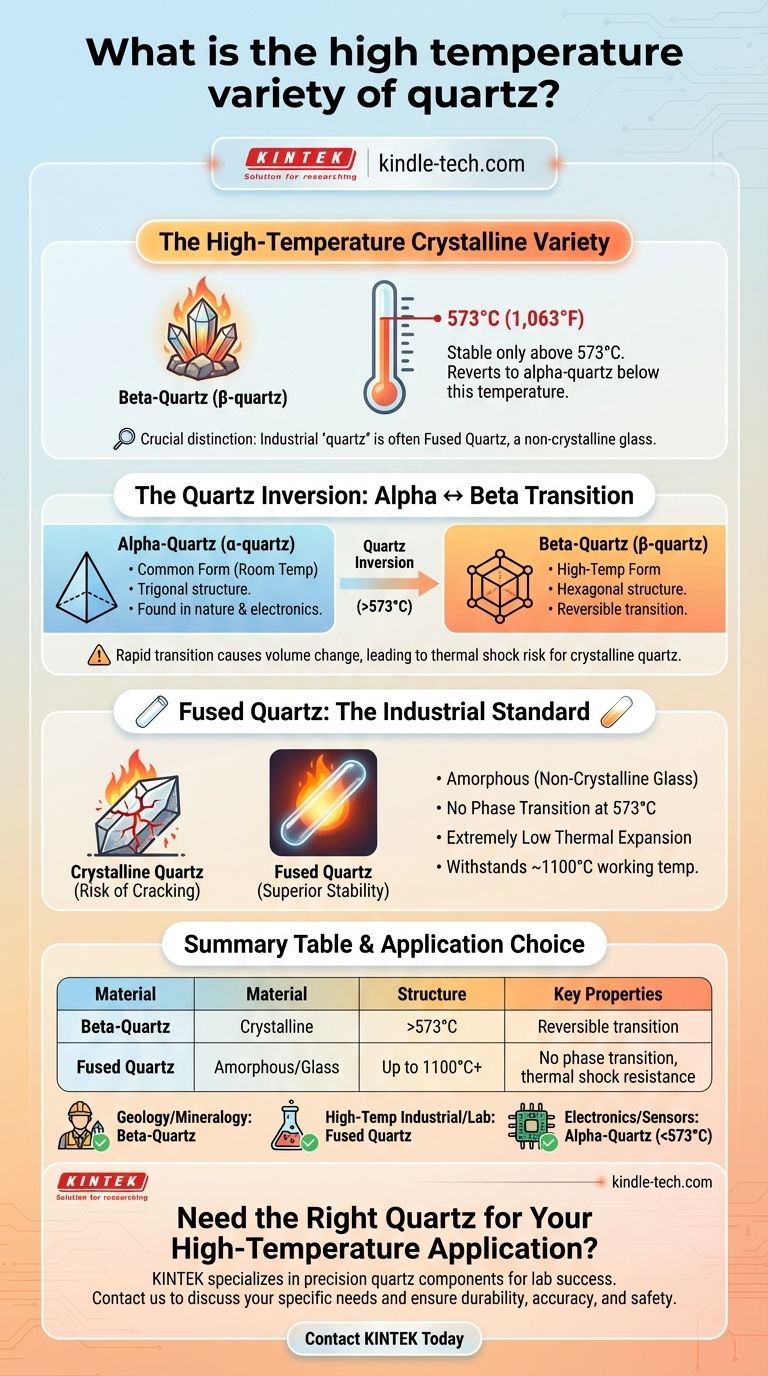
At its core, the high-temperature crystalline variety of quartz is known as beta-quartz (β-quartz). This form is stable only above 573°C (1,063°F) at atmospheric pressure. Below this temperature, it reverts to the common alpha-quartz (α-quartz) we find in nature.
The term "quartz" is often used for two distinct materials. While beta-quartz is the high-temperature mineral, the "quartz" used in industrial applications like high-temperature tubes is actually fused quartz, a non-crystalline glass with a much higher working temperature. Understanding this distinction is critical.

The Two Forms of Crystalline Quartz
Crystalline quartz (silicon dioxide, SiO₂) is a polymorph, meaning it can exist in different crystal structures depending on temperature and pressure. The two most common forms are alpha- and beta-quartz.
Alpha-Quartz (α-quartz): The Common Form
Alpha-quartz is the form of quartz stable at room temperature and normal atmospheric pressure. It possesses a trigonal crystal structure. This is the quartz found in granite, sand, and used in most electronic applications for its piezoelectric properties.
Beta-Quartz (β-quartz): The High-Temperature Form
Above 573°C, the atomic structure of alpha-quartz rearranges into a more symmetrical hexagonal crystal structure. This new arrangement is beta-quartz. It is slightly less dense than alpha-quartz.
The Quartz Inversion: A Reversible Transition
The transformation from alpha- to beta-quartz at 573°C is called the quartz inversion. This change is rapid and completely reversible.
As beta-quartz cools back down below 573°C, it immediately transforms back into alpha-quartz. This is why we do not find natural beta-quartz crystals at the Earth's surface.
Fused Quartz vs. Crystalline Quartz: The Industrial Reality
The reference to a quartz tube withstanding 1100°C introduces a crucial point. This tube is not made of crystalline beta-quartz, but of an entirely different material often conflated with it.
What is Fused Quartz?
Fused quartz, also called fused silica or quartz glass, is an amorphous (non-crystalline) form of silicon dioxide. It is manufactured by melting extremely high-purity crystalline quartz (like sand) at temperatures around 2000°C and then cooling it.
Why Fused Quartz Excels at High Temperatures
This material is the standard for high-temperature labware and industrial components for two key reasons:
- No Phase Transition: As a glass, it has no crystal structure and therefore does not undergo the disruptive alpha-beta inversion at 573°C.
- Extremely Low Thermal Expansion: It barely expands or contracts with changes in temperature, making it incredibly resistant to thermal shock.
A working temperature of 1100°C is standard for fused quartz, which has a softening point above 1600°C.
Understanding the Trade-offs
Choosing between crystalline and fused quartz depends entirely on the application, as their behaviors at high temperatures are fundamentally different.
Crystalline Quartz: The Risk of Thermal Shock
The quartz inversion involves a sudden change in volume. Heating or cooling a piece of crystalline quartz too quickly through the 573°C transition point will cause it to crack or shatter. This makes it unsuitable for applications involving rapid temperature changes.
Fused Quartz: Superior Stability, Different Properties
The primary advantage of fused quartz is its exceptional thermal stability and resistance to thermal shock. However, because it is not crystalline, it does not have the piezoelectric properties of alpha-quartz, making it useless for applications in timing and frequency control.
Making the Right Choice for Your Goal
To select the correct material, you must be clear about your objective.
- If your primary focus is geology or mineralogy: The high-temperature polymorph is beta-quartz, which exists only above the 573°C inversion point.
- If your primary focus is a high-temperature industrial or lab application: The material you need is fused quartz (fused silica), an amorphous glass valued for its thermal stability.
- If your primary focus is electronics or sensors: You need alpha-quartz for its piezoelectric properties and must operate it well below the 573°C transition temperature.
Ultimately, success depends on choosing the right form of silicon dioxide for the specific thermal and physical demands of your task.
Summary Table:
| Material | Structure | Stable Temperature Range | Key Properties |
|---|---|---|---|
| Beta-Quartz (β-quartz) | Crystalline | >573°C (1063°F) | High-temperature mineral, reversible transition |
| Fused Quartz (Quartz Glass) | Amorphous (Glass) | Up to 1100°C+ | No phase transition, superior thermal shock resistance |
Need the Right Quartz for Your High-Temperature Application?
Choosing between crystalline quartz for its piezoelectric properties and fused quartz for extreme thermal stability is critical for your lab's success. KINTEK specializes in high-performance lab equipment and consumables, including precision quartz components. Our experts can help you select the ideal material to ensure durability, accuracy, and safety in your processes.
Contact us today via our contact form to discuss your specific needs and discover how KINTEK's solutions can enhance your laboratory's efficiency and reliability.
Visual Guide
Related Products
- High Temperature Resistant Optical Quartz Glass Sheet
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 400-700nm Wavelength Anti Reflective AR Coating Glass
People Also Ask
- What is the working temperature of quartz glass? Master Its High-Temp Limits & Applications
- What is the high temperature of quartz? Key Thresholds for Crystalline vs. Fused Silica
- What is optical quartz? The Ultimate Material for UV and High-Temp Optics
- Does quartz have a high melting point? Discover Its Superior High-Temperature Performance
- What temperature does quartz glass melt at? Understanding Its Softening Point and Practical Limits

















