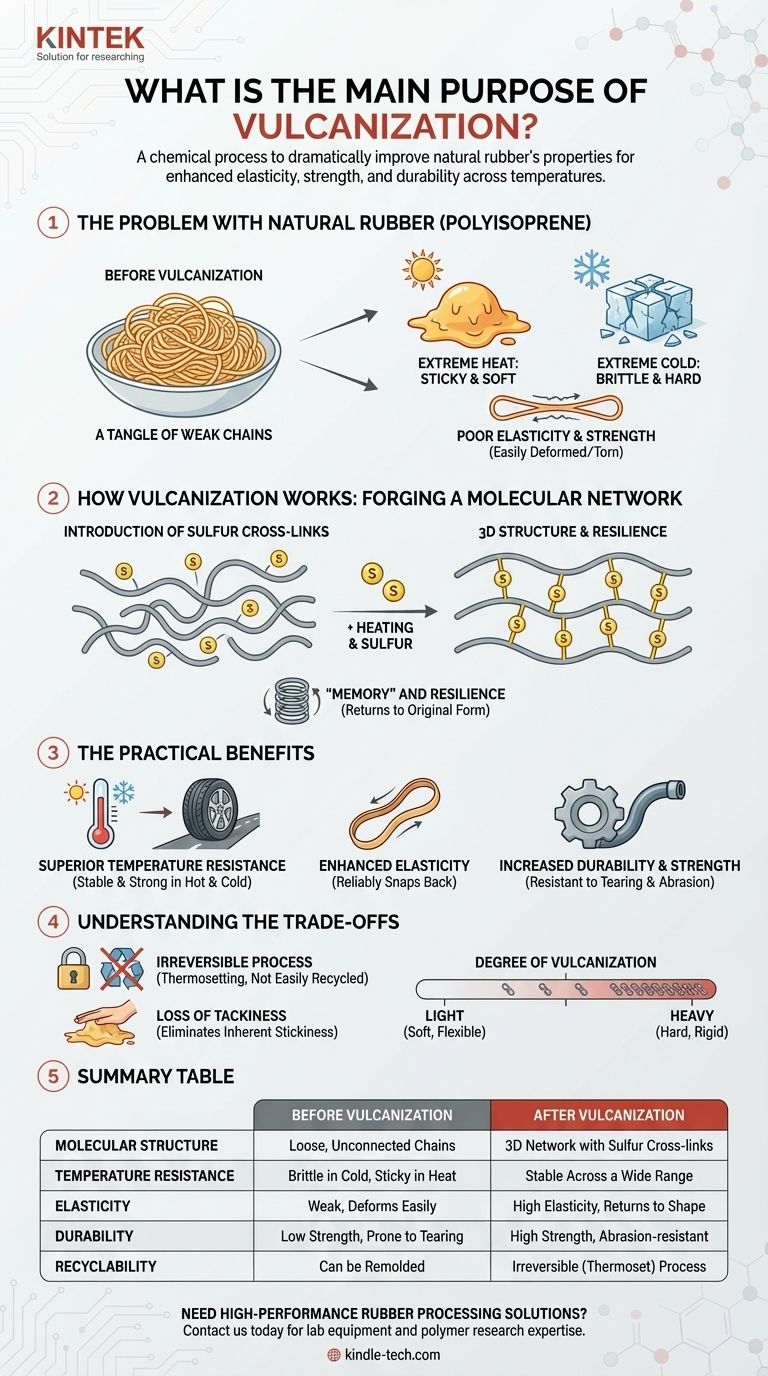
In essence, vulcanization is a chemical process designed to dramatically improve the physical properties of natural rubber. Its primary purpose is to enhance elasticity, strength, and durability, especially across a wide range of temperatures where untreated rubber would become sticky when hot or brittle when cold.
Natural rubber in its raw form is a weak, sticky material with limited practical use. The core purpose of vulcanization is to introduce chemical cross-links between its polymer chains, transforming it from a flawed natural substance into a stable, strong, and highly elastic engineering material.

The Problem with Natural Rubber
Before vulcanization, natural rubber is a polymer called polyisoprene. While it has some elastic properties, it suffers from several critical flaws that make it unsuitable for most applications.
A Tangle of Weak Chains
Imagine natural rubber as a bowl of cooked spaghetti. The long, individual polymer chains are entangled but not chemically bonded to each other. They can slide past one another with relative ease.
Extreme Temperature Vulnerability
This weak structure makes raw rubber highly sensitive to temperature. When heated, the chains move more freely, causing the rubber to become soft and sticky. In the cold, the chains lock up, making the material hard and brittle.
Poor Elasticity and Strength
When you stretch raw rubber, the polymer chains uncoil and slide apart. Because there are no strong connections to pull them back, the material does not return to its original shape perfectly and can be permanently deformed or easily torn.
How Vulcanization Works: Forging a Molecular Network
Vulcanization permanently solves these issues by fundamentally changing the rubber's molecular structure. The process was famously discovered by Charles Goodyear in 1839.
Introducing Sulfur Cross-Links
The most common method involves heating natural rubber with sulfur. During this process, the sulfur atoms form strong covalent bonds, or cross-links, between the individual polyisoprene chains.
From Chains to a 3D Structure
These cross-links act like bridges, tying all the separate polymer chains together into a single, massive, three-dimensional network. The rubber is no longer a collection of individual strands but a unified molecular structure.
The Result: "Memory" and Resilience
This network structure gives the rubber "memory." When the material is stretched, the chains can still uncoil, but the cross-links prevent them from sliding apart permanently. When the stretching force is released, these cross-links pull the chains back to their original positions, resulting in excellent elasticity.
The Practical Benefits of Vulcanized Rubber
This molecular transformation translates into several crucial real-world advantages that make rubber one of the most versatile materials available.
Superior Temperature Resistance
Because the polymer chains are locked in place, vulcanized rubber remains strong and flexible in both hot and cold conditions. This is why a car tire can function effectively on a hot summer road and a freezing winter day.
Enhanced Elasticity
Vulcanized rubber can undergo significant deformation and will reliably snap back to its original form. This property is essential for everything from rubber bands to shock absorbers.
Increased Durability and Strength
The cross-linked network makes the material much stronger and more resistant to tearing, abrasion, and chemical attack. It transforms a fragile substance into a material that can withstand immense physical stress.
Understanding the Trade-offs
While overwhelmingly beneficial, the vulcanization process does introduce certain trade-offs that are important to recognize.
A Non-Reversible Process
Vulcanization is a thermosetting process, meaning it is irreversible. Once the cross-links are formed, the rubber cannot be melted down and remolded like a thermoplastic. This makes recycling more complex.
Loss of "Tackiness"
Natural rubber is inherently sticky, a property known as tack. While this is usually undesirable, it is useful for certain applications like adhesives. Vulcanization eliminates this tackiness.
The Degree of Vulcanization
The properties of the final product depend heavily on the number of sulfur cross-links. Light vulcanization with less sulfur creates a soft, flexible material like a rubber band. Heavy vulcanization creates a hard, rigid material like a hockey puck.
Making the Right Choice for Your Goal
Understanding the purpose of vulcanization helps you select the correct material for your specific engineering or design challenge.
- If your primary focus is a strong, all-weather, and highly elastic material (like a tire or hose): Vulcanization is not just beneficial, it is absolutely essential to achieve the required performance.
- If your primary focus is a material that can be easily remolded or recycled: You should consider a thermoplastic elastomer (TPE), which mimics rubber but can be melted and reprocessed.
- If your primary focus is an adhesive where stickiness is key: You may use unvulcanized natural rubber or a different type of polymer altogether.
By chemically locking polymer chains together, vulcanization transforms a weak natural substance into one of the most essential and reliable materials of the modern world.
Summary Table:
| Aspect | Before Vulcanization | After Vulcanization |
|---|---|---|
| Molecular Structure | Loose, unconnected polymer chains | 3D network with sulfur cross-links |
| Temperature Resistance | Brittle in cold, sticky in heat | Stable across a wide temperature range |
| Elasticity | Weak, deforms easily | High elasticity, returns to shape |
| Durability | Low strength, prone to tearing | High strength, abrasion-resistant |
| Recyclability | Can be remolded | Irreversible (thermoset) process |
Need high-performance rubber processing solutions for your lab or production line? KINTEK specializes in lab equipment and consumables for material testing and polymer research. Whether you're developing new rubber compounds or optimizing vulcanization parameters, our expertise can help you achieve precise, reliable results. Contact us today to discuss how we can support your laboratory's material science needs!
Visual Guide
Related Products
- Anti-Cracking Press Mold for Lab Use
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- What are the advantages of using high-strength graphite molds in the hot press sintering of Ti6Al4V-based composites?
- What are the specific functions of graphite molds in the vacuum hot-press sintering process? Expert Insights for Ceramics
- What role do high-temperature pressure molds play in SiCp/Al fabrication? Enhancing Densification and Thermal Uniformity
- What role does a high-purity graphite mold play during hot pressing? Optimize Boron Carbide Sintering at 1850°C
- What is the role of graphite molds during the hot pressing of LSLBO ceramics? Essential for High-Density Electrolytes



















