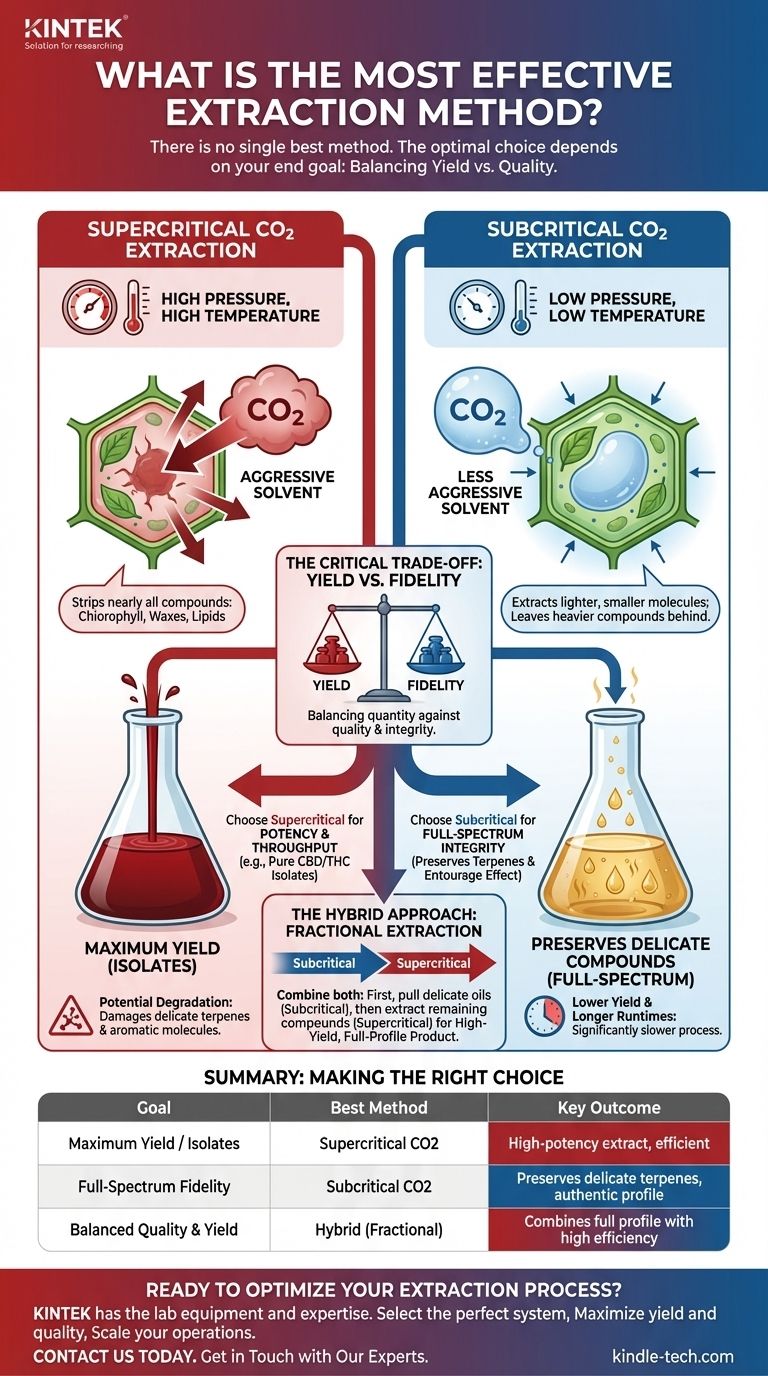
There is no single "most effective" extraction method. The optimal choice depends entirely on your end goal, as different methods excel at producing different results. The two primary CO2 extraction techniques, subcritical and supercritical, are defined by their operating temperature and pressure, which fundamentally changes how they interact with plant material.
The core trade-off in extraction is between yield and quality. Supercritical CO2 extraction maximizes yield and efficiency, making it ideal for isolates. Subcritical CO2 extraction preserves the full profile of delicate compounds, making it superior for high-fidelity, full-spectrum products.

Understanding Supercritical CO2 Extraction
Supercritical extraction uses carbon dioxide at a high temperature and high pressure. This forces the CO2 into a "supercritical" state, where it has properties of both a liquid and a gas simultaneously.
The Principle: High Pressure, High Temperature
In its supercritical state, CO2 becomes a highly effective and aggressive solvent. It can easily penetrate raw plant material and dissolve larger, heavier compounds like chlorophyll, waxes, and omega-3 and omega-6 lipids.
The Result: Maximum Yield
This method is known for its efficiency and high yields. Because of its aggressive solvent properties, it strips the plant material of nearly all its available compounds in a relatively short amount of time.
The Downside: Potential for Degradation
The high temperatures and pressures required for supercritical extraction can damage or destroy more delicate, volatile compounds. Terpenes and other aromatic molecules are particularly susceptible to degradation in this process, meaning the final extract may not reflect the full chemical profile of the original plant.
Understanding Subcritical CO2 Extraction
Subcritical extraction uses carbon dioxide at a low temperature and lower pressure. This keeps the CO2 in a liquid state, altering its solvent properties significantly compared to the supercritical method.
The Principle: Low Pressure, Low Temperature
In its liquid subcritical state, CO2 is a much less aggressive solvent. It tends to extract only the lighter, smaller molecules from the plant material, leaving the heavier compounds and waxes behind.
The Result: Preserving Delicate Compounds
This gentle process is ideal for preserving temperature-sensitive compounds. It is the preferred method for creating extracts rich in volatile terpenes and flavonoids, resulting in a product that more accurately represents the plant's original aromatic and chemical profile.
The Downside: Lower Yield and Longer Runtimes
Because subcritical extraction is less aggressive, it produces a lower overall yield compared to a supercritical run on the same material. The process is also significantly slower, requiring more time to extract the target compounds.
The Critical Trade-off: Yield vs. Fidelity
Choosing a method requires a clear understanding of your priorities. You are almost always balancing the quantity of the final product against the quality and integrity of its chemical profile.
Choose Supercritical for Potency and Throughput
If your goal is to produce a high-potency isolate (e.g., pure CBD or THC) or to maximize the sheer volume of extract from your starting material, supercritical is the more effective method. Its speed and power are unmatched for this purpose.
Choose Subcritical for Full-Spectrum Integrity
If you are creating a premium, full-spectrum or broad-spectrum product where the preservation of terpenes and the "entourage effect" is the primary goal, subcritical is the superior choice. The final product will have a more robust and nuanced aromatic profile.
The Hybrid Approach: Fractional Extraction
Many expert extractors use a combination of both methods. They first perform a subcritical run to pull out the delicate terpenes and light oils. Then, they run the same plant material through a supercritical process to extract the remaining heavier compounds. The two resulting extracts can then be combined to create a high-yield product that still contains the full, rich profile of the original plant.
Making the Right Choice for Your Goal
To select the best method, you must first define your desired outcome.
- If your primary focus is maximum yield and creating isolates: Supercritical extraction is the most efficient and powerful method for this goal.
- If your primary focus is preserving the full profile of delicate terpenes and flavonoids: Subcritical extraction is the only method that can reliably achieve this level of quality.
- If your primary focus is a "best of both worlds" approach for a high-quality, high-yield product: A two-stage fractional extraction, starting subcritical and finishing supercritical, will deliver the most comprehensive results.
Ultimately, the most "effective" extraction method is the one that best serves the intention behind your final product.
Summary Table:
| Goal | Best Method | Key Outcome |
|---|---|---|
| Maximum Yield / Isolates | Supercritical CO2 | High-potency extract, efficient |
| Full-Spectrum Fidelity | Subcritical CO2 | Preserves delicate terpenes, authentic profile |
| Balanced Quality & Yield | Hybrid (Fractional) | Combines full profile with high efficiency |
Ready to Optimize Your Extraction Process?
The right equipment is crucial for achieving your target product profile. Whether you need the high-throughput power of supercritical extraction or the delicate precision of a subcritical system, KINTEK has the lab equipment and expertise to support your goals.
We help you:
- Select the perfect system for isolates, full-spectrum, or hybrid extraction.
- Maximize your yield and product quality with reliable, precision equipment.
- Scale your operations with consumables and support for your laboratory needs.
Contact us today to discuss your specific application and find the most effective solution for your lab.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Benchtop Laboratory Freeze Dryer for Lab Use
People Also Ask
- Why is a precision vibrating sieve shaker essential for metal leaching research? Optimize Your Particle Size Analysis
- What is the function of sieving equipment in CuAlMn alloys? Master Pore Size Precision
- What is the role of standard sieves in gold scrap leaching kinetic studies? Ensure Precision in Particle Classification
- What is the primary purpose of using standard sieves? Master Particle Uniformity for High-Quality Catalyst Preparation
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis



















