The most common process gas for sputtering is Argon (Ar). Its primary role is not to be part of the final material, but to act as the physical mechanism for deposition. In a vacuum chamber, the argon is ionized to create a plasma, and these ions are accelerated to bombard a target, physically knocking off atoms that then deposit as a thin film onto a substrate.
The choice of a process gas is a critical parameter that defines the nature of the sputtering process. While inert gases like Argon facilitate a purely physical deposition, reactive gases like Oxygen or Nitrogen are intentionally used to chemically create specific compound films during deposition.
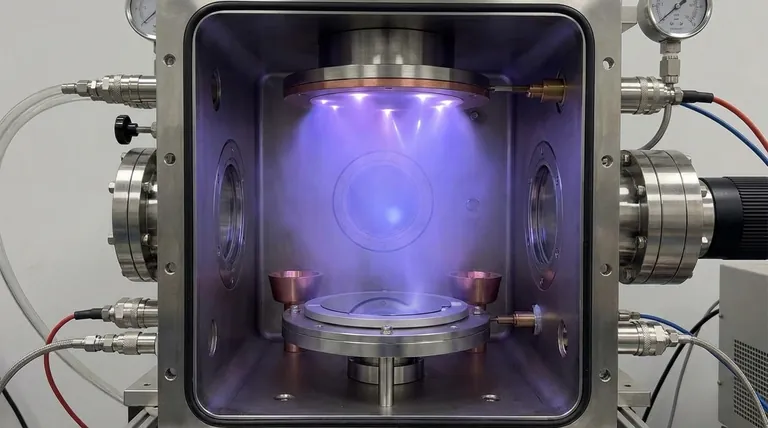
The Fundamental Role of Process Gas
To understand why certain gases are chosen, you must first understand what the gas actually does in the sputtering chamber. The process is a physical chain of events.
Creating the Plasma
A process gas is introduced into a low-pressure vacuum chamber. An electric field is applied, which strips electrons from the gas atoms. This creates a mixture of positively charged ions and free electrons known as plasma.
The Bombardment Mechanism
The sputtering target (the material you want to deposit) is given a negative electrical charge. This attracts the positively charged gas ions from the plasma, causing them to accelerate and collide with the target at high velocity.
Momentum Transfer is Key
The collision is a purely physical process based on momentum transfer. The high-energy gas ion strikes the target and transfers its kinetic energy to the target atoms, dislodging them from the surface. This is the "sputtering" effect.
Deposition onto the Substrate
These ejected, or "sputtered," neutral atoms from the target travel through the chamber and land on the substrate, building up layer by layer to form a thin film.
Why Argon is the Standard Choice
Argon is the default process gas for most sputtering applications for several clear reasons.
It's Chemically Inert
As a noble gas, Argon does not readily react with other elements. This is crucial because it ensures the sputtering process is purely physical. The deposited film will have the same chemical composition as the target material, without unwanted chemical reactions.
Favorable Atomic Mass
For efficient sputtering, the atomic weight of the process gas ion should be reasonably close to that of the target atoms to maximize momentum transfer. Argon's atomic mass (39.9 u) provides a good balance for a wide range of common target materials.
Cost and Availability
Argon is the most abundant noble gas in Earth's atmosphere, making it significantly more affordable and readily available than other inert options like Krypton or Xenon.
When to Use Other Gases
While Argon is the workhorse, specific goals require different process gases. The choice is always driven by the desired outcome, whether it's efficiency or the final film's chemical composition.
Other Inert Gases (Ne, Kr, Xe)
To optimize the sputtering rate, the atomic mass of the gas should be matched to the target.
- Neon (Ne) is lighter than Argon and is sometimes used for sputtering very light target elements for more efficient energy transfer.
- Krypton (Kr) and Xenon (Xe) are heavier. Their high molecular weight results in a more powerful bombardment, leading to higher sputtering and deposition rates, especially for heavy target materials.
Reactive Gases (O₂, N₂)
Sometimes, the goal is not to deposit a pure material but to create a compound. This is called reactive sputtering. In this process, a reactive gas is intentionally introduced into the chamber along with the inert gas.
The reactive gas combines with the sputtered atoms either in transit or on the substrate surface. This allows for the deposition of films that are different from the target material, such as oxides, nitrides, or oxynitrides. For example, one could sputter a pure Titanium target in an atmosphere containing Oxygen to deposit a Titanium Dioxide (TiO₂) film.
Understanding the Trade-offs
Choosing a process gas involves balancing performance, cost, and the desired properties of the final film.
Sputtering Rate vs. Cost
Using heavier inert gases like Krypton or Xenon can significantly increase deposition rates, which is valuable in high-volume manufacturing. However, these gases are substantially more expensive than Argon, creating a direct trade-off between throughput and operational cost.
Film Purity vs. Desired Composition
Using an inert gas is essential when the goal is a high-purity film that chemically matches the target. In contrast, reactive sputtering intentionally sacrifices this purity to create a specific compound, turning the process from purely physical to chemo-physical.
Process Control Complexity
Reactive sputtering is a more complex process to control. The precise ratio of inert gas to reactive gas must be carefully managed to achieve the correct film stoichiometry (the chemical ratio of elements). Incorrect control can lead to inconsistent film properties or undesirable effects on the target itself.
Making the Right Choice for Your Goal
The selection of a process gas is a deliberate choice tied directly to the application's requirements.
- If your primary focus is depositing a pure, elemental film: Use an inert gas. Argon is the universal starting point, but consider Neon for very light targets or Krypton/Xenon for heavy targets to optimize the deposition rate.
- If your primary focus is creating a specific compound film (like an oxide or nitride): You must use reactive sputtering, introducing a gas like oxygen or nitrogen along with an inert gas like Argon.
- If your primary focus is cost-effectiveness for general applications: Argon almost always provides the best balance of performance, versatility, and low cost.
Ultimately, the process gas is a fundamental control parameter used to tailor the sputtering process to produce a specific, desired material.
Summary Table:
| Gas Type | Primary Use Case | Key Characteristics |
|---|---|---|
| Argon (Ar) | General-purpose sputtering of pure films | Inert, cost-effective, good atomic mass balance |
| Krypton (Kr) / Xenon (Xe) | Sputtering heavy target materials | Heavier inert gases, higher sputtering rates |
| Oxygen (O₂) / Nitrogen (N₂) | Reactive sputtering for compound films (oxides, nitrides) | Chemically reactive to form compounds with target material |
Ready to Optimize Your Sputtering Process?
Choosing the right process gas is critical for achieving the desired film properties, deposition rate, and cost-efficiency for your specific application. Whether you need to deposit pure elemental films or complex compounds, KINTEK's expertise in lab equipment and consumables can help you select the ideal sputtering setup.
Our team specializes in providing solutions for laboratory thin-film deposition needs. Contact us today to discuss how we can support your research or production goals with the right equipment and consumables.
Visual Guide
Related Products
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Engineering Advanced Fine Ceramics Aluminium Oxide Al2O3 Ceramic Washer for Wear-Resistant Applications
People Also Ask
- How is deposition time calculated? Mastering the Clock for Strategic Legal Advantage
- What is electron beam coating? A Guide to High-Performance PVD Thin Films
- What is gold sputtered? A Guide to High-Purity Vacuum Coating for Electronics & SEM
- What is sputter coating used for? Achieve Superior Thin Films for Electronics, Optics, and Tools
- What are sputtering systems used for? A Guide to Advanced Thin-Film Deposition













