In X-Ray Fluorescence (XRF) analysis, the radiation originates from one of two primary sources: an X-ray tube or a radioactive isotope. While both are capable of producing the high-energy photons needed for analysis, virtually all modern XRF instruments, from handheld units to large laboratory systems, rely on miniaturized X-ray tubes. This is because tubes offer superior control, performance, and safety.
The source of radiation in an XRF analyzer is a controlled device designed to bombard a sample with high-energy X-rays. Understanding how this source works is the key to grasping the capabilities, limitations, and safety considerations of any XRF instrument.

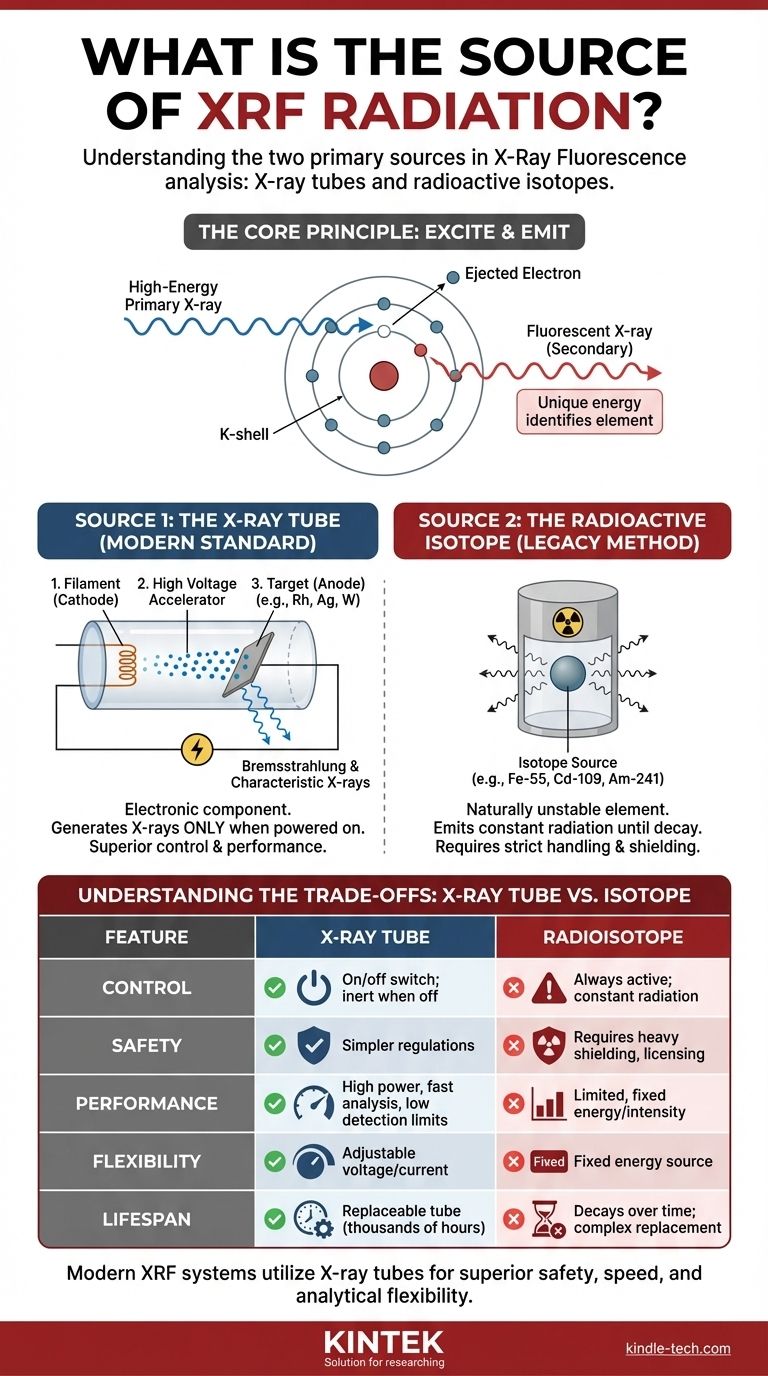
The Core Principle: How an XRF Source Works
The Goal: Exciting the Sample's Atoms
The fundamental job of the XRF source is to emit a stream of primary X-rays with enough energy to interact with the atoms in your material sample.
When a high-energy primary X-ray strikes an atom in the sample, it can knock an electron out of one of its inner orbital shells (e.g., the K or L shell). This creates a vacancy, leaving the atom in an unstable, excited state.
The Fluorescence Process
To return to a stable state, an electron from a higher-energy outer shell immediately drops down to fill the vacancy. This transition releases a specific amount of energy in the form of a secondary, or "fluorescent," X-ray.
The energy of this fluorescent X-ray is unique to the element from which it was emitted. The XRF analyzer's detector measures the energies and number of these fluorescent X-rays to determine the elemental composition of the sample.
The Two Primary Types of XRF Sources
While the goal is the same, the method of generating the initial X-rays differs significantly between the two source technologies.
Source 1: The X-ray Tube (The Modern Standard)
An X-ray tube is an electronic component that generates X-rays only when powered on. Think of it as a specialized, high-power light bulb, but one that emits X-rays instead of visible light.
The process involves three key parts:
- A filament (cathode) is heated, releasing a cloud of electrons.
- A high voltage is applied, accelerating these electrons at immense speed towards a target.
- A target (anode), made of a specific pure metal like rhodium (Rh), silver (Ag), or tungsten (W), is struck by the electrons.
This impact causes the electrons to decelerate rapidly, producing a broad spectrum of X-rays known as Bremsstrahlung radiation. It also excites the atoms of the target material itself, adding the target's own characteristic X-rays to the beam, which can be highly efficient for exciting certain elements in the sample.
Source 2: The Radioactive Isotope (The Legacy Method)
Some older or highly specialized XRF analyzers use a radioactive isotope as the excitation source. These are single elements, such as Iron-55 (Fe-55), Cadmium-109 (Cd-109), or Americium-241 (Am-241), that are naturally unstable.
As these isotopes decay, they emit gamma rays or X-rays at specific, fixed energies. This radiation is constant and cannot be turned off; the source is always active until it fully decays. The intensity of the radiation decreases predictably over time according to the isotope's half-life.
Understanding the Trade-offs: Why X-ray Tubes Dominate
The shift from radioisotopes to X-ray tubes is not arbitrary; it is driven by major advantages in performance, safety, and flexibility.
Control and Safety
This is the most critical difference. An X-ray tube produces radiation only when it is powered on. When the power is off, it is completely inert and emits no radiation.
A radioisotope source is always on. It emits radiation 24/7, requiring heavy shielding, strict licensing, security protocols for storage and transport, and complex disposal procedures. This makes X-ray tube systems vastly simpler from a regulatory and safety standpoint.
Performance and Speed
X-ray tubes can be driven at a much higher power, producing a significantly greater quantity of X-rays (higher flux). This intense beam excites the sample more effectively, leading to faster analysis times and the ability to measure elements at much lower concentrations (lower detection limits).
Analytical Flexibility
With an X-ray tube, the operator can adjust the voltage and current. This allows the primary X-ray beam to be optimized for exciting different groups of elements. For example, a lower voltage is better for light elements, while a higher voltage is needed for heavy metals. This flexibility is impossible with a fixed-energy radioisotope source.
Lifespan and Maintenance
An X-ray tube has a finite operational life, typically several thousand hours, after which it is easily and safely replaced. A radioisotope's intensity decays based on its half-life, requiring frequent recalibration and an eventual complex, highly regulated, and expensive source replacement and disposal process.
Making the Right Choice for Your Application
The best source technology is dictated entirely by your analytical requirements and operational constraints.
- If your primary focus is high performance, speed, and analytical flexibility: A modern system with an X-ray tube is the only logical choice for detecting trace elements or analyzing a wide range of materials.
- If your primary focus is safety and regulatory simplicity: An X-ray tube-based system is unequivocally the superior choice, as it generates no radiation when powered off and is subject to far less stringent regulations.
- If you are analyzing a limited set of known elements: While an older radioisotope-based unit could perform the task, a modern tube-based system will do it faster, more precisely, and with far fewer logistical burdens.
Understanding the source of radiation empowers you to not only select the right instrument but also to interpret your analytical results with greater confidence.
Summary Table:
| Feature | X-ray Tube (Modern Standard) | Radioisotope (Legacy Method) |
|---|---|---|
| Control | On/off switch; radiation only when powered | Always active; constant radiation |
| Safety | Inert when off; simpler regulations | Requires heavy shielding, licensing, and secure disposal |
| Performance | High power, fast analysis, low detection limits | Limited, fixed energy and intensity |
| Flexibility | Adjustable voltage/current for different elements | Fixed energy source |
| Lifespan | Replaceable tube (thousands of hours) | Decays over time (half-life); complex replacement |
Ready to choose the right XRF analyzer for your lab? KINTEK specializes in high-performance lab equipment, including XRF systems with advanced X-ray tube technology. Our instruments offer superior safety, speed, and flexibility for your elemental analysis needs. Contact our experts today to find the perfect solution for your laboratory!
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Optical Window Glass Substrate Wafer CaF2 Substrate Window Lens
- Laboratory Multifunctional Small Speed-Adjustable Horizontal Mechanical Shaker for Lab
People Also Ask
- What are the advantages of using Extremely fast Joule Heating (EJH) equipment? Precision in Thin Film Synthesis
- What is the thermal conductivity coefficient of graphite? A Guide to Its Anisotropic Properties
- What role does drying or curing equipment play in NSHPC synthesis? Ensuring Structural Precision in Porous Carbons
- What are water baths used for? Achieve Precise & Gentle Temperature Control for Your Lab Samples
- What are the methods of bio-oil upgrade? Transform Unstable Bio-Oil into Valuable Fuel
- What is the difference between chamber and membrane filter press? Optimize Your Solid-Liquid Separation
- Is pyrolysis oil harmful? Understanding the Risks of This Reactive Biofuel
- What are the grades of graphite? A Practical Guide to Choosing the Right Material for Your Application















