In essence, a single-wall carbon nanotube (SWCNT) is a seamless, hollow cylinder formed by rolling a single-atom-thick sheet of graphene. The structure is composed entirely of carbon atoms arranged in a hexagonal honeycomb lattice. This fundamental architecture, inherited from graphene, is responsible for the nanotube's extraordinary properties.
The most critical structural detail is not just that it's a rolled-up sheet, but how it is rolled. This "twist," known as chirality, dictates the nanotube's diameter, atomic arrangement, and most importantly, its fundamental electronic properties.
The Foundation: From Graphene to Nanotube
To truly understand an SWCNT's structure, we must start with its building block: a sheet of graphene.
The Graphene Lattice
Graphene is a one-atom-thick layer of carbon atoms bonded together in a honeycomb pattern. These bonds are known as sp2-hybridized bonds, the same type found in graphite, and are exceptionally strong.
The Concept of the Roll-up Vector
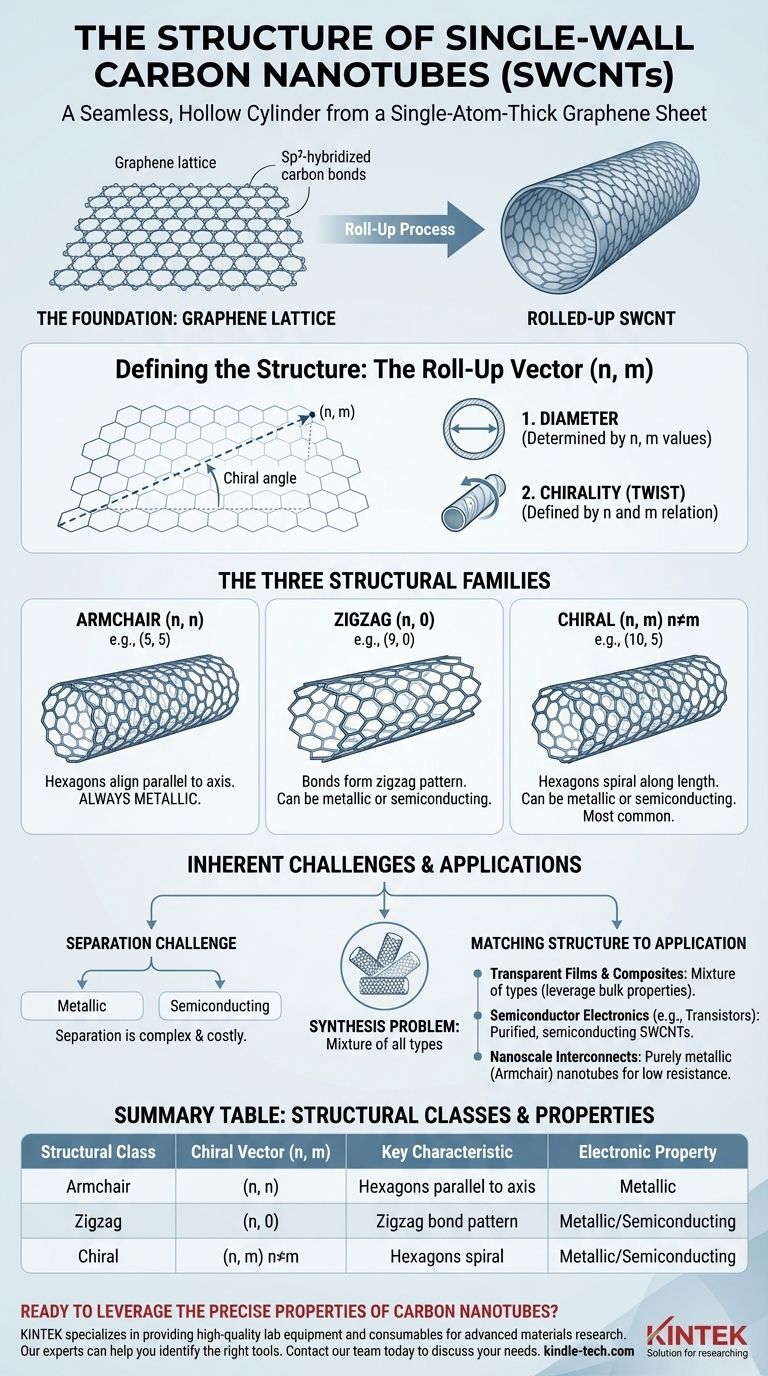
Imagine taking that flat sheet of graphene and rolling it into a tube. The angle at which you roll it determines the pattern of the hexagons along the seam of the tube.
This "roll-up" is defined by a mathematical concept called the chiral vector, denoted by a pair of integers (n, m). These indices specify which two points on the graphene lattice are joined together to form the cylinder's circumference.
How (n, m) Defines the Structure
The (n, m) indices are the unique blueprint for every SWCNT. They precisely define two key physical attributes:
- Diameter: The values of n and m directly determine the diameter of the nanotube.
- Chirality (Twist): The relationship between n and m defines the chiral angle, or the degree of twist in the hexagonal lattice as it wraps around the tube.
The Three Classes of SWCNT Structures
Based on their (n, m) indices, all single-wall carbon nanotubes fall into one of three distinct structural families.
Armchair Nanotubes (n, n)
When the indices are identical (e.g., (5, 5) or (10, 10)), the resulting structure is called armchair. The hexagonal rings are aligned perfectly parallel to the tube's axis, creating a pattern that resembles an armrest along the circumference.
Zigzag Nanotubes (n, 0)
When the second index is zero (e.g., (9, 0) or (12, 0)), the nanotube has a zigzag structure. The pattern of carbon bonds forms a distinct zigzag shape along the circumference of the tube.
Chiral Nanotubes (n, m)
This is the most general case, where n ≠ m and m ≠ 0 (e.g., (10, 5)). These chiral nanotubes have a visible twist, with the hexagons spiraling down the length of the tube at a specific angle. They are the most common type found in real-world synthesis.
Understanding the Inherent Challenges
The direct link between atomic structure and properties creates a significant hurdle in nanotube applications.
The Synthesis Problem
Current large-scale synthesis methods, like chemical vapor deposition (CVD), inevitably produce a mixture of all three types of SWCNTs. The output is a blend of armchair, zigzag, and chiral tubes with a wide distribution of diameters.
The Separation Challenge
This structural diversity means that any raw sample contains both metallic and semiconducting nanotubes. For high-performance electronics, these must be separated, a complex and costly process that remains a major focus of materials research.
Matching Structure to Your Application
The specific (n, m) structure you need is entirely dependent on your end goal.
- If your primary focus is transparent conductive films or high-strength composites: A mixture of SWCNT types is often sufficient, as you are leveraging the average bulk properties of the material.
- If your primary focus is semiconductor electronics like transistors: You must use highly purified, semiconducting SWCNTs, making the isolation of specific chiral or zigzag types absolutely critical.
- If your primary focus is creating nanoscale electrical interconnects: The ideal structure would be purely metallic (armchair) nanotubes to achieve the lowest possible electrical resistance.
Ultimately, understanding the atomic structure of a carbon nanotube is the key to harnessing its unparalleled technological potential.
Summary Table:
| Structural Class | Chiral Vector (n, m) | Key Characteristic | Electronic Property |
|---|---|---|---|
| Armchair | (n, n) | Hexagons aligned parallel to tube axis | Metallic (always) |
| Zigzag | (n, 0) | Carbon bonds form a zigzag pattern | Can be metallic or semiconducting |
| Chiral | (n, m) n≠m | Hexagons spiral along the tube length | Can be metallic or semiconducting |
Ready to leverage the precise properties of carbon nanotubes in your research or product development? The specific (n, m) structure of an SWCNT directly determines its electronic behavior, making material selection critical for applications in electronics, composites, and more. KINTEK specializes in providing high-quality lab equipment and consumables for advanced materials research. Our experts can help you identify the right tools for your nanotube synthesis, characterization, and application challenges.
Contact our team today to discuss how we can support your laboratory's specific needs.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application



















