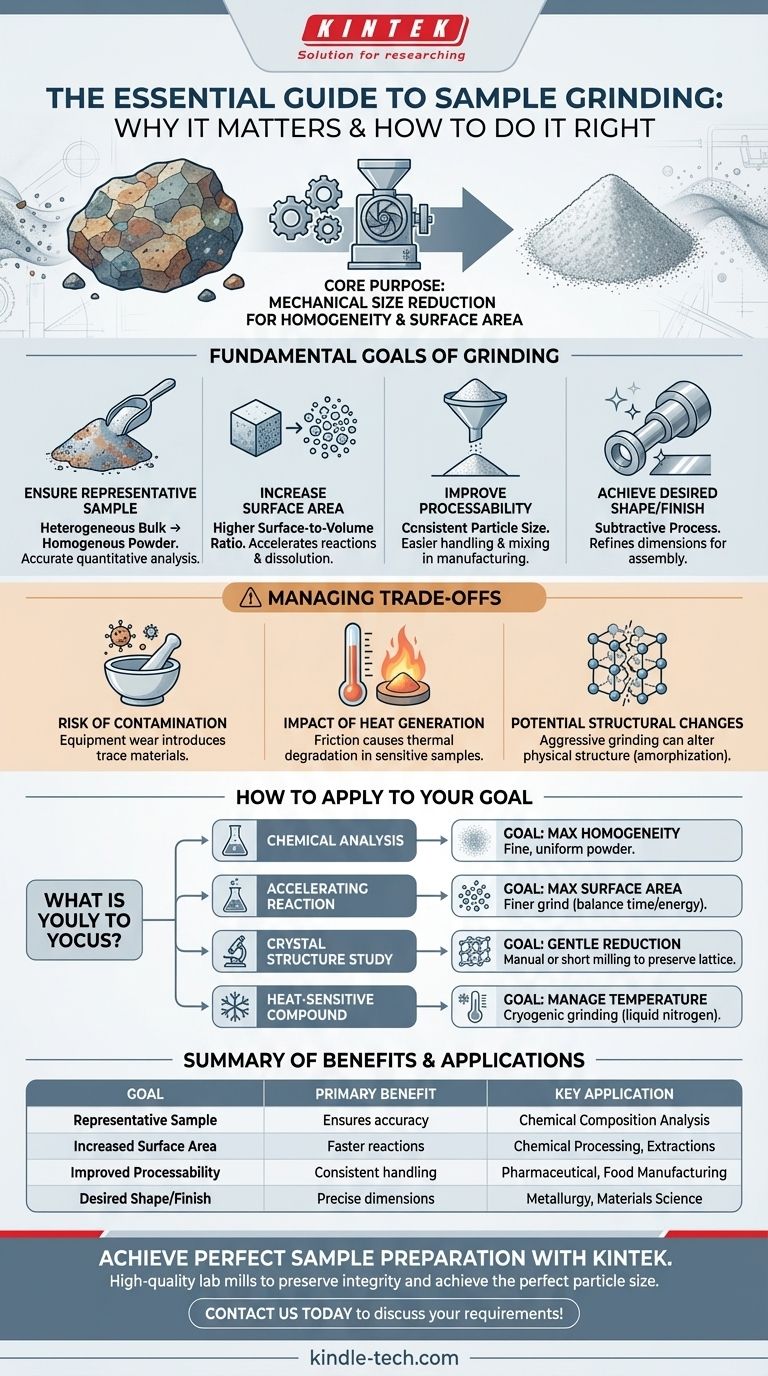
At its core, grinding is a necessary process of mechanical size reduction. It breaks down a larger, often heterogeneous bulk sample into smaller, more uniform particles. This is done not just to make something smaller, but to fundamentally alter its physical properties to enable, improve, or standardize subsequent processing or analysis.
The primary reason for grinding a sample is to increase its homogeneity and surface area. This ensures that any small portion taken for analysis is representative of the whole and that the material can react or dissolve more efficiently in subsequent steps.

The Fundamental Goals of Grinding
While the action of grinding is simple, its objectives are critical for a wide range of scientific and industrial processes. The end goal is rarely just "smaller pieces," but rather the specific properties that result from that size reduction.
To Ensure a Representative Sample
Most bulk materials are heterogeneous, meaning their composition is not uniform throughout. For example, a rock may have veins of different minerals, or a plant leaf has different structures.
Grinding and mixing these components creates a homogenous powder. This ensures that a small scoop of the powder has the same average composition as the entire original sample, which is essential for accurate quantitative analysis.
To Increase Surface Area
Breaking a large particle into many smaller ones dramatically increases the total surface-area-to-volume ratio. Think of a single sugar cube versus the same amount of granulated sugar.
This increased surface area is critical for accelerating processes like chemical reactions, dissolution, and extraction. More surface means more contact points for a solvent or reactant to do its work, leading to faster and more complete results.
To Improve Processability and Handling
Uniform powders are often much easier to handle, transport, and measure than large, irregular objects. In food processing or pharmaceutical manufacturing, consistent particle size ensures that powders flow correctly through machinery, mix evenly, and produce a consistent final product.
To Achieve a Desired Shape or Finish
In fields like metallurgy and materials science, grinding is a subtractive process used to remove unwanted material from a surface. This refines the object's shape, achieves precise dimensions, or creates a specific surface finish, as seen when preparing metal parts for assembly.
Understanding the Trade-offs
Grinding is a powerful but aggressive process. It is not without potential downsides that must be managed to ensure the integrity of the sample.
The Risk of Contamination
The grinding equipment itself—whether it's a mortar and pestle, a ball mill, or an industrial grinder—can wear down. This can introduce trace amounts of the grinding material (e.g., agate, steel, zirconia) into your sample, acting as a contaminant that can interfere with sensitive analyses.
The Impact of Heat Generation
Grinding generates significant friction and, therefore, heat. For heat-sensitive (thermolabile) samples, such as many biological molecules or organic compounds, this can cause thermal degradation, altering or destroying the very substance you intend to study.
The Potential for Structural Changes
Overly aggressive grinding can do more than just reduce size; it can impart enough energy to change a material's physical structure. For example, it can damage or even destroy the crystalline lattice of a material, a process known as amorphization, which would render an analysis like X-ray diffraction useless.
How to Apply This to Your Goal
The right grinding strategy depends entirely on what you plan to do with the sample afterward.
- If your primary focus is accurate chemical analysis: Your goal is maximum homogeneity. Grind until you have a fine, uniform powder to ensure any subsample is representative of the whole.
- If your primary focus is accelerating a reaction or dissolution: Your goal is maximum surface area. A finer grind is generally better, but balance this against the time and energy required.
- If your primary focus is studying a material's crystal structure: Your goal is gentle size reduction. Use manual grinding or shorter milling times to avoid damaging the crystalline lattice.
- If your primary focus is processing a heat-sensitive compound: Your goal is to manage temperature. Use methods like cryogenic grinding (using liquid nitrogen) to keep the sample frozen and prevent degradation.
Ultimately, understanding the purpose of grinding transforms it from a rote task into a critical step for ensuring the quality and accuracy of your results.
Summary Table:
| Goal of Grinding | Key Benefit | Primary Application |
|---|---|---|
| Representative Sample | Ensures homogeneity for accurate analysis | Chemical composition analysis |
| Increased Surface Area | Accelerates reactions, dissolution, and extraction | Chemical processing, extractions |
| Improved Processability | Creates uniform powders for consistent handling | Pharmaceutical, food manufacturing |
| Desired Shape/Finish | Refines material dimensions and surface | Metallurgy, materials science |
Achieve perfect sample preparation with the right equipment from KINTEK.
Grinding is a critical first step that dictates the success of your entire analysis or process. Using the wrong technique or equipment can lead to contamination, heat degradation, and inaccurate results.
KINTEK specializes in high-quality lab mills and grinding equipment designed to preserve sample integrity while achieving the perfect particle size for your specific application—whether it's for chemical analysis, materials science, or pharmaceutical development.
Let our experts help you select the ideal grinding solution for your lab's needs. Contact us today to discuss your requirements and ensure your sample preparation is a solid foundation for success!
Visual Guide
Related Products
- Laboratory Single Horizontal Jar Mill
- Mini Planetary Ball Mill Machine for Laboratory Milling
- High Energy Planetary Ball Mill Machine for Laboratory Horizontal Tank Type
- High Energy Planetary Ball Mill Milling Machine for Laboratory
- High Energy Planetary Ball Mill Milling Machine for Laboratory
People Also Ask
- What is the mechanism of action of a colloid mill? Master High-Shear Processing for Superior Emulsions and Dispersions
- Why are mechanical grinding or high-shear mixing processes necessary? Achieve Uniform Zinc Anode Protective Layers
- What advantages does a high energy nano mill offer over a traditional ball mill? Optimize Your LiFePO4 Cathode Quality
- How does a laboratory ball mill prepare catalysts like CuAlO2? Enhancing Efficiency with Mechanical Alloying
- What is the name of the lab equipment used for grinding? Choose the Right Mill for Your Sample
- Why grinding is important in laboratory techniques? Ensure Accurate & Reproducible Results
- What kind of material is a hammer mill used for? Process Brittle, Dry, and Crystalline Materials Efficiently
- Why are high-shear mixing or ultrasonic homogenizers necessary for MMT nanocomposites? Unlock True Nano-Reinforcement



















