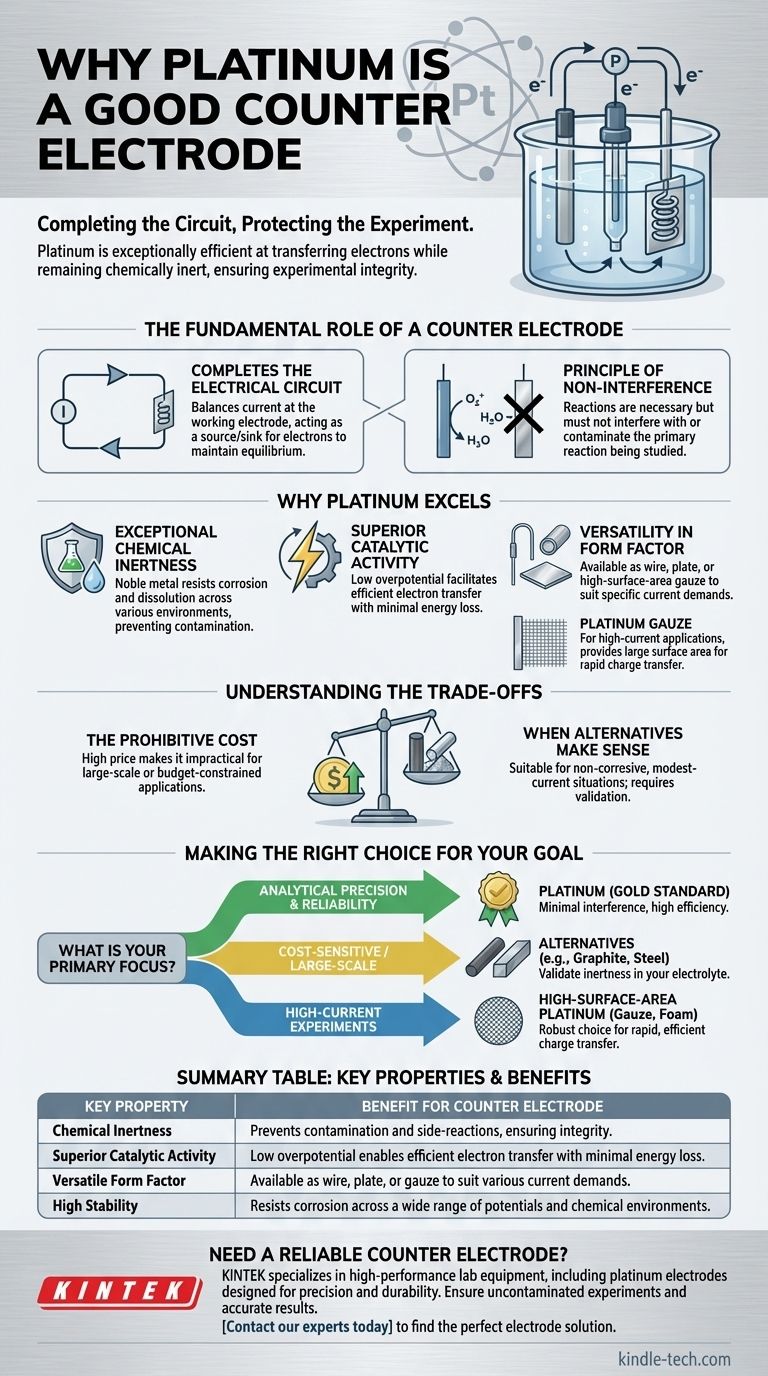
Platinum is an excellent counter electrode because it is exceptionally efficient at transferring electrons while remaining chemically inert in most electrochemical systems. This unique combination ensures it completes the electrical circuit necessary for an experiment without interfering with the chemical reaction you are trying to measure.
The primary job of a counter electrode is to balance the current of the working electrode, effectively completing the circuit. Its quality is therefore defined by its ability to perform this electrical function efficiently without introducing any chemical side-reactions that could contaminate or alter the experiment.

The Fundamental Role of a Counter Electrode
To understand why platinum is so effective, we must first define the role of the counter electrode (also known as the auxiliary electrode). It exists for one reason: to provide a pathway for current to flow so that the desired reaction can occur at the working electrode.
Completing the Electrical Circuit
In any electrochemical cell, the current flowing at the working electrode must be balanced. The counter electrode serves as the other end of this circuit, acting as a source or sink for electrons to keep the system in electrical equilibrium.
The Principle of Non-Interference
The reactions occurring at the counter electrode are a necessary consequence of passing current, but they are not the focus of the experiment. It is critical that these reactions, and any products they generate, do not interfere with the primary reaction being studied at the working electrode.
Why Platinum Excels in This Role
Platinum's material properties make it nearly ideal for fulfilling the counter electrode's function. It balances high performance with stability, ensuring the focus remains on the working electrode.
Exceptional Chemical Inertness
Platinum is a noble metal, meaning it resists corrosion and dissolution across a wide range of chemical environments and electrical potentials. This inertness is its most critical feature, as it prevents the electrode itself from reacting and releasing ions that could contaminate your solution and invalidate your results.
Superior Catalytic Activity
A counter electrode must pass current efficiently. Platinum is an excellent catalyst, meaning it facilitates the transfer of electrons with minimal energy loss. This property, known as low overpotential, ensures the voltage applied by the potentiostat is primarily used to drive the reaction at the working electrode, not wasted on forcing the counter reaction.
Versatility in Form Factor
As a material, platinum is stable and can be easily manufactured into various shapes suitable for electrode construction. It is commonly available as a wire, plate, or high-surface-area gauze. This allows for the construction of counter electrodes tailored to the specific current demands of an experiment. For high-current applications, a platinum gauze provides a large surface area to handle the charge transfer without issue.
Understanding the Trade-offs
While platinum is often the default choice, it is not perfect for every situation. Objectivity requires acknowledging its significant drawback.
The Prohibitive Cost
Platinum's primary disadvantage is its high cost. As a precious metal, it is far more expensive than other potential electrode materials. This can make it impractical for large-scale industrial applications, disposable sensors, or budget-constrained teaching laboratories.
When Alternatives Make Sense
In many situations where the electrolyte is non-corrosive and the current demands are modest, less expensive materials can function perfectly well. Materials like graphite rods or even stainless steel can serve as suitable counter electrodes, provided you have verified they are inert under your specific experimental conditions.
Making the Right Choice for Your Goal
Selecting a counter electrode is a practical decision that balances performance requirements against constraints like cost and scale.
- If your primary focus is analytical precision and reliability: Platinum is the gold standard, and its cost is justified by the assurance of minimal interference and high efficiency.
- If your primary focus is cost-sensitive applications or large-scale setups: Investigate alternatives like graphite or stainless steel, but first validate their inertness in your specific electrolyte.
- If your primary focus is high-current experiments: A high-surface-area platinum electrode, such as a gauze or foam, is the most robust choice to facilitate rapid and efficient charge transfer.
Ultimately, understanding these principles allows you to select a counter electrode that serves its purpose: enabling your experiment, not defining it.
Summary Table:
| Key Property | Benefit for Counter Electrode |
|---|---|
| Chemical Inertness | Prevents contamination and side-reactions, ensuring experimental integrity |
| Superior Catalytic Activity | Low overpotential enables efficient electron transfer with minimal energy loss |
| Versatile Form Factor | Available as wire, plate, or gauze to suit various current demands |
| High Stability | Resists corrosion across a wide range of potentials and chemical environments |
Need a reliable counter electrode for your electrochemical research? KINTEK specializes in high-performance lab equipment, including platinum electrodes designed for precision and durability. Our products ensure your experiments remain uncontaminated and your results are accurate. Contact our experts today to find the perfect electrode solution for your laboratory needs.
Visual Guide
Related Products
- Platinum Auxiliary Electrode for Laboratory Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Gold Disc Electrode
People Also Ask
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data
- How should a platinum mesh electrode be operated during an experiment? Ensure Accurate Electrochemical Measurements
- Why is a glassy carbon disc electrode an indispensable consumable? Ensure Reliable Catalyst Evaluation Today
- Why are screen-printed carbon electrodes (SPCE) commonly selected for PB/PEI characterization? Key Efficiency Benefits
- What parameters require monitoring during an experiment involving a carbon fiber brush? Ensure Reliable Results
- What is the step-by-step process for polishing, testing, and cleaning an electrode? A Pro Guide for Precision Results
- What are the correct storage conditions for an RVC sheet? Ensure Long-Term Performance and Integrity
- What pretreatment steps should be taken before using an electrode holder? Ensure Reliable Electrochemical Measurements



















