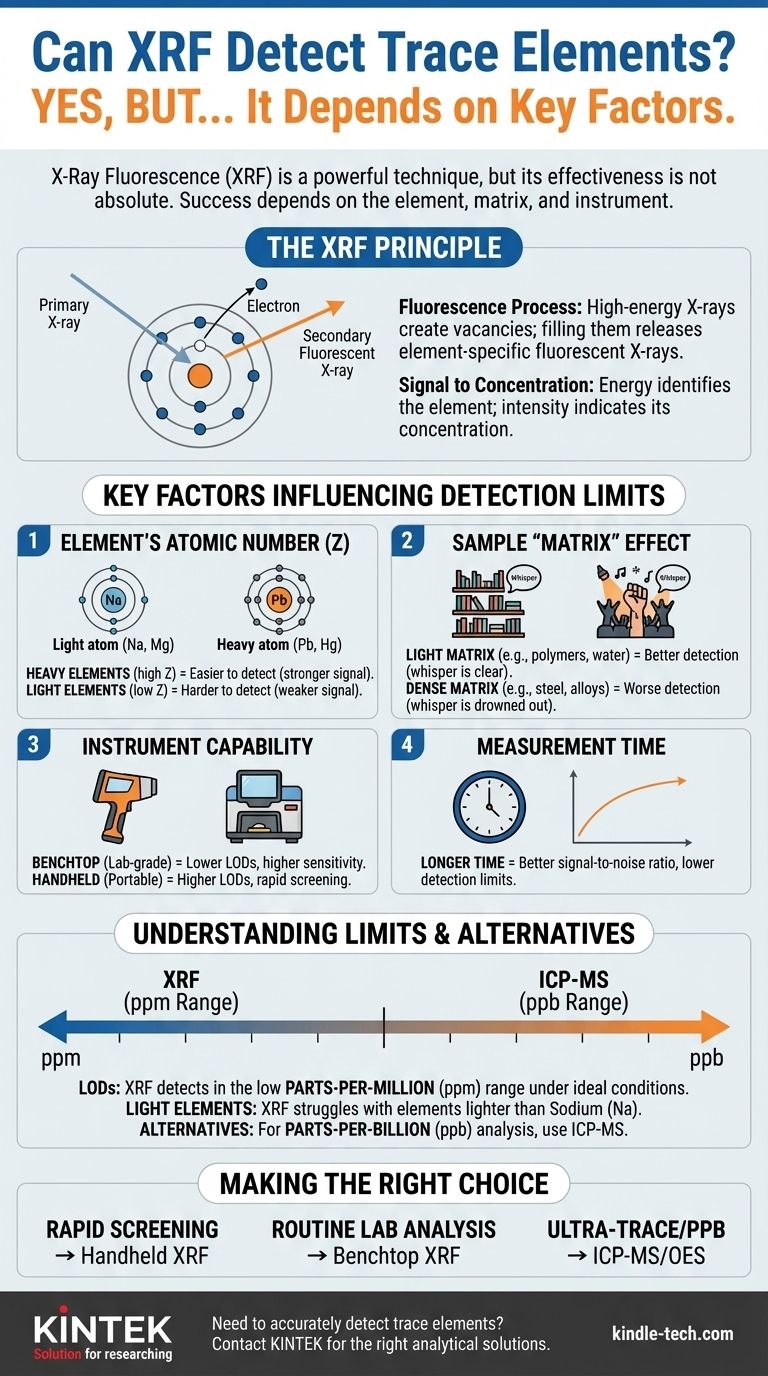
Yes, X-Ray Fluorescence (XRF) is a widely used and powerful technique for detecting trace elements. Its effectiveness, however, is not absolute. The ability of an XRF analyzer to detect a specific trace element depends critically on the element itself, the surrounding material (the "matrix"), and the quality of the instrument being used.
The central issue is not if XRF can detect trace elements, but under what conditions it is the right tool for the job. Success depends on aligning the instrument's capabilities with the specific element of interest, the sample composition, and the detection limits your application requires.

The Principle: How XRF Identifies Elements
To understand XRF's capabilities, you must first grasp its fundamental mechanism. This process is non-destructive, making it a highly valued analytical method.
The "Fluorescence" Process
An XRF instrument directs a beam of primary X-rays at a sample. This high-energy beam strikes atoms within the sample, knocking an electron out of a low-energy, inner orbital.
This creates an unstable vacancy. To regain stability, an electron from a higher-energy, outer orbital immediately drops down to fill the empty spot. This transition releases a specific amount of energy in the form of a secondary (or "fluorescent") X-ray.
From Signal to Concentration
The energy of this fluorescent X-ray is a unique signature of the element it came from. The instrument's detector measures both the energy (to identify the element) and the number of fluorescent X-rays (the intensity). The intensity of the signal is directly proportional to the concentration of that element in the sample.
Key Factors That Determine Detection Limits
Whether a "trace" amount is detectable is a function of separating its faint signal from the background noise. Several factors control this signal-to-noise ratio.
The Element's Atomic Number (Z)
Heavier elements (those with a higher atomic number, like lead or mercury) are generally easier to detect at trace levels. They produce higher-energy fluorescent X-rays that are more easily distinguished from background noise.
Conversely, light elements (like sodium, magnesium, and aluminum) produce very low-energy fluorescent X-rays. These signals are easily absorbed by the air or the sample itself and are much harder to differentiate from the background, resulting in higher (worse) detection limits.
The Sample "Matrix" Effect
The "matrix" is everything in the sample that is not the element you are trying to measure. This is often the most significant factor in trace analysis.
Imagine trying to hear a whisper (the trace element) in different environments. A light, low-density matrix (like a polymer or water) is like a quiet library—the whisper is easy to hear. A heavy, dense matrix (like a steel alloy) is like a loud rock concert—the whisper is drowned out by the noise and interference of the surrounding crowd. This absorption of the signal by the matrix is known as the "matrix effect."
The Instrument's Capability
Not all XRF analyzers are created equal. The hardware plays a decisive role in achieving low detection limits.
- Handheld vs. Benchtop: Laboratory-grade benchtop systems (like WDXRF or high-power EDXRF) are far more sensitive than portable, handheld XRF (pXRF) analyzers. They have more powerful X-ray tubes and superior detectors.
- Detector Technology: Modern Silicon Drift Detectors (SDDs) offer excellent resolution, which helps separate the peaks of trace elements from those of major elements.
- Atmosphere Modification: For light elements, benchtop systems can use a vacuum or a helium purge to remove air, which would otherwise absorb the weak fluorescent signals.
The Measurement Time
XRF analysis is a statistical process of counting photons. A longer measurement time allows the detector to collect more characteristic X-rays from the trace element, improving the signal-to-noise ratio and lowering the limit of detection. Doubling the measurement time does not halve the detection limit, but it does improve it.
Understanding the Trade-offs and Limitations
While powerful, XRF is not the solution for every analytical problem. Objectivity requires knowing its boundaries.
Limits of Detection (LODs)
Under ideal conditions (a light matrix, a heavy element, and a long measurement on a lab system), XRF can achieve detection limits in the low parts-per-million (ppm) range, sometimes even sub-ppm.
For handheld analyzers in the field, LODs are typically higher, often in the range of 5 to 50 ppm, depending on the element and matrix. XRF is generally not suitable for analysis at the parts-per-billion (ppb) level.
The Light Element Challenge
As mentioned, elements lighter than sodium (Na) are extremely difficult for most XRF systems to quantify accurately. Elements like carbon, nitrogen, and oxygen are beyond the capabilities of standard XRF.
When Other Methods Are Better
For ultimate trace performance, other techniques are superior.
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry) is the gold standard for ultra-trace analysis, routinely measuring in the ppb and even parts-per-trillion (ppt) ranges. It is the correct choice when the lowest possible detection is required.
- AAS (Atomic Absorption Spectroscopy) is another excellent technique, particularly for measuring one or a few specific elements in liquid samples at very low concentrations.
Making the Right Choice for Your Application
The decision to use XRF should be based on a clear understanding of your analytical goal.
- If your primary focus is rapid screening and sorting in the field: Handheld XRF is the ideal tool for its speed and portability, perfect for identifying materials or checking for restricted substances like lead or cadmium.
- If your primary focus is high-quality process control or routine lab analysis at ppm levels: A benchtop EDXRF or WDXRF system offers the precision and stability needed for reliable quality assurance in a production environment.
- If your primary focus is quantifying sub-ppm (ppb) concentrations or analyzing complex liquid samples: Techniques like ICP-MS or ICP-OES are required, as they offer significantly lower detection limits than XRF.
By understanding these factors, you can confidently determine whether XRF is the appropriate and most effective tool for your specific analytical challenge.
Summary Table:
| Factor | Impact on Trace Detection |
|---|---|
| Element Atomic Number | Heavier elements (e.g., Pb) are easier to detect than light elements (e.g., Na). |
| Sample Matrix | Light matrices (e.g., polymers) offer better detection than dense matrices (e.g., steel). |
| Instrument Type | Benchtop systems (WD/EDXRF) have lower detection limits than handheld XRF analyzers. |
| Measurement Time | Longer analysis times improve the signal-to-noise ratio, lowering detection limits. |
| Typical Detection Limit | Parts-per-million (ppm) range; not suitable for parts-per-billion (ppb) analysis. |
Need to accurately detect trace elements in your materials?
KINTEK specializes in laboratory equipment and consumables, providing the right analytical solutions for your specific needs. Whether you require the rapid screening capability of a handheld XRF or the high precision of a benchtop system for quality control, our experts can help you select the ideal instrument to achieve reliable, ppm-level detection limits.
Let us help you enhance your lab's capabilities. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
People Also Ask
- What pressure should KBr pellets be? Optimize Your FTIR Spectroscopy with the Right Load
- What is the application of pressure in a hydraulic press? Harness Force Multiplication for Your Lab or Industry
- How does a laboratory hydraulic press contribute to electrolyte layers in solid-state batteries? Achieve Peak Density
- What is the difference between hydraulic and mechanical press machines? Choose the Right Force for Your Production
- What is the purpose of applying high pressure in dry cathode preparation? Achieve Peak Solid-State Battery Density
- Why is pressing force important in sintering? Achieve Denser, Stronger Materials Faster
- How is a uniaxial hydraulic press utilized in the formation of solid-state electrolyte pellets? Boost Ionic Conductivity
- What is a hydraulic forging press used for? Harnessing Controlled Power for Complex Metal Forming








