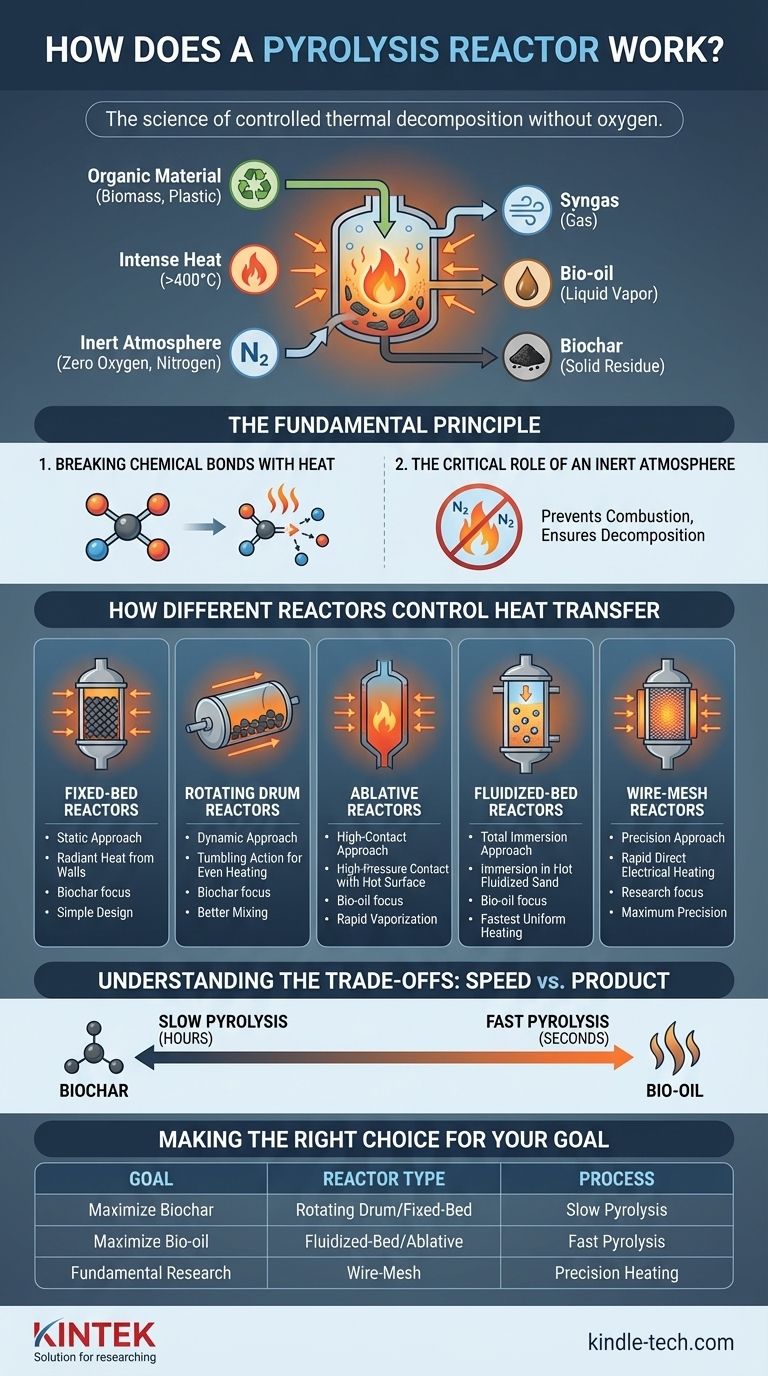
At its core, a pyrolysis reactor is a specialized vessel designed to do one thing: apply intense heat to organic material in a completely oxygen-free environment. This process doesn't burn the material; instead, it uses thermal energy to break down complex molecules into simpler, more valuable products like bio-oil, biochar, and syngas. It is a system of controlled thermal decomposition.
The key to understanding how different pyrolysis reactors work is to focus on one variable: the method of heat transfer. The specific design of a reactor—whether it tumbles, fluidizes, or presses the feedstock—is engineered to control the speed and uniformity of heating, which directly determines the final products.

The Fundamental Principle: Decomposition Without Oxygen
A pyrolysis reactor's function is rooted in basic thermodynamic principles. To be effective, it must master two environmental conditions: high heat and zero oxygen.
Breaking Chemical Bonds with Heat
Every organic material, from wood chips to plastic waste, is made of long, complex molecules held together by chemical bonds. A pyrolysis reactor's primary job is to supply enough thermal energy—often at temperatures exceeding 400°C—to shatter these bonds. This disintegration breaks the large molecules down into smaller, lighter molecules that can be collected as gases (syngas) and condensable vapors (bio-oil), leaving behind a solid, carbon-rich residue (biochar).
The Critical Role of an Inert Atmosphere
The absence of oxygen is non-negotiable. If oxygen were present, the organic material would simply combust, or burn, releasing its energy as heat and light. By creating an inert (non-reactive) atmosphere, often by flushing the chamber with a gas like nitrogen, the reactor ensures that thermal decomposition is the only reaction that can occur.
How Different Reactors Control Heat Transfer
The engineering differences between reactor types are all about solving the challenge of transferring heat into the feedstock efficiently and uniformly.
The Static Approach: Fixed-Bed Reactors
This is the simplest design. The organic material, or substrate, is loaded onto a stationary bed at the bottom of the vessel. Heat is then applied to the reactor's exterior walls and slowly radiates inward. This is a relatively slow and less uniform method of heat transfer, as the material in the center is heated much later than the material touching the walls.
The Dynamic Approach: Rotating Drum Reactors
A rotating drum (or rotary kiln) reactor improves on the fixed-bed design. The feedstock is placed inside a large, cylindrical drum that is continuously rotated as it is heated externally in a furnace. This tumbling motion constantly mixes the material, ensuring a more even and consistent exposure to the hot inner walls of the drum.
The High-Contact Approach: Ablative Reactors
Ablative pyrolysis uses pressure and friction to achieve rapid heat transfer. In this design, the biomass is pressed with significant force against a very hot moving surface. The intense, direct contact causes the material to "melt" and vaporize almost instantly, leaving a thin film of oil that helps lubricate the process for subsequent particles.
The Total Immersion Approach: Fluidized-Bed Reactors
This is one of the most efficient designs for rapid heat transfer. The reactor contains a bed of fine material, such as sand, which is heated. An inert gas is then forced up through the bottom of the bed, causing the hot sand particles to bubble and behave like a fluid. When the feedstock is introduced, it is instantly immersed in this hot, churning fluid, ensuring every particle is heated uniformly and almost instantaneously.
The Precision Approach: Wire-Mesh Reactors
Used almost exclusively for laboratory research, a wire-mesh reactor offers maximum precision. A very small sample is clamped between two metal grids (the mesh), which are then heated extremely rapidly. This setup minimizes secondary reactions and allows researchers to precisely study the initial moments of decomposition, making it invaluable for scientific investigation but impractical for large-scale production.
Understanding the Trade-offs: Speed vs. Product
The rate of heat transfer is the single most important factor influencing the final product yields. This is the trade-off at the heart of reactor design.
Slow Pyrolysis (Hours)
Reactors that heat material slowly, like fixed-bed and rotating drum designs, give the molecules time to rearrange and form stable, carbon-rich structures. This process maximizes the production of biochar.
Fast Pyrolysis (Seconds)
Reactors that transfer heat almost instantly, such as fluidized-bed and ablative reactors, shock the material. The molecules are vaporized so quickly they don't have time to form char. This process maximizes the yield of condensable vapors, which form liquid bio-oil.
Simplicity vs. Efficiency
A simple fixed-bed reactor is relatively easy and cheap to build but offers poor control and efficiency. In contrast, a fluidized-bed reactor is complex and expensive but provides the superior heat transfer necessary for high-yield bio-oil production.
Making the Right Choice for Your Goal
The ideal reactor is determined entirely by your desired end product and operational scale.
- If your primary focus is maximizing biochar production: A slow pyrolysis reactor like a rotating drum or fixed-bed design is your most effective choice.
- If your primary focus is maximizing liquid bio-oil yield: A fast pyrolysis reactor, such as a fluidized-bed or ablative system, is necessary for its rapid heat transfer capabilities.
- If your primary focus is fundamental research and analysis: A wire-mesh reactor offers the precise control needed to study the initial stages of thermal decomposition.
Ultimately, understanding that reactor design is simply a tool to control heat transfer empowers you to select the right process for the right product.
Summary Table:
| Reactor Type | Primary Heating Method | Best For Product | Key Characteristic |
|---|---|---|---|
| Fixed-Bed | Slow, radiant heat from walls | Biochar | Simple, low-cost design |
| Rotating Drum | Tumbling action for even heating | Biochar | Better mixing than fixed-bed |
| Ablative | High-pressure contact with hot surface | Bio-oil | Rapid vaporization |
| Fluidized-Bed | Immersion in hot, fluidized sand | Bio-oil | Fastest, most uniform heating |
| Wire-Mesh | Rapid, direct electrical heating | Research | Maximum precision for lab studies |
Ready to select the right pyrolysis reactor for your specific biomass conversion goals? The experts at KINTEK are here to help. Whether your focus is maximizing biochar for soil enhancement or producing high-yield bio-oil for energy, we provide the advanced lab equipment and consumables you need for efficient and effective thermal processing. Contact our team today to discuss your project requirements and discover how KINTEK's solutions can optimize your pyrolysis outcomes.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- Why are there chains inside a rotary kiln? Boost Efficiency & Control with Internal Heat Exchange
- How long is a cement rotary kiln? Optimizing Length for Maximum Efficiency & Output
- What is the temperature of a rotary kiln? It's a Controlled Thermal Journey, Not a Single Number
- What are the advantages of rotary kiln furnace? Achieve Superior Uniformity & Efficiency
- What are the principles of a rotary kiln? Master the Mechanics of High-Temperature Processing
- What are the different zones in a rotary kiln? A Guide to Precise Thermal Processing
- What is pyrolysis of biomass to biofuel? A Complete Guide to Converting Waste into Liquid Fuel



















