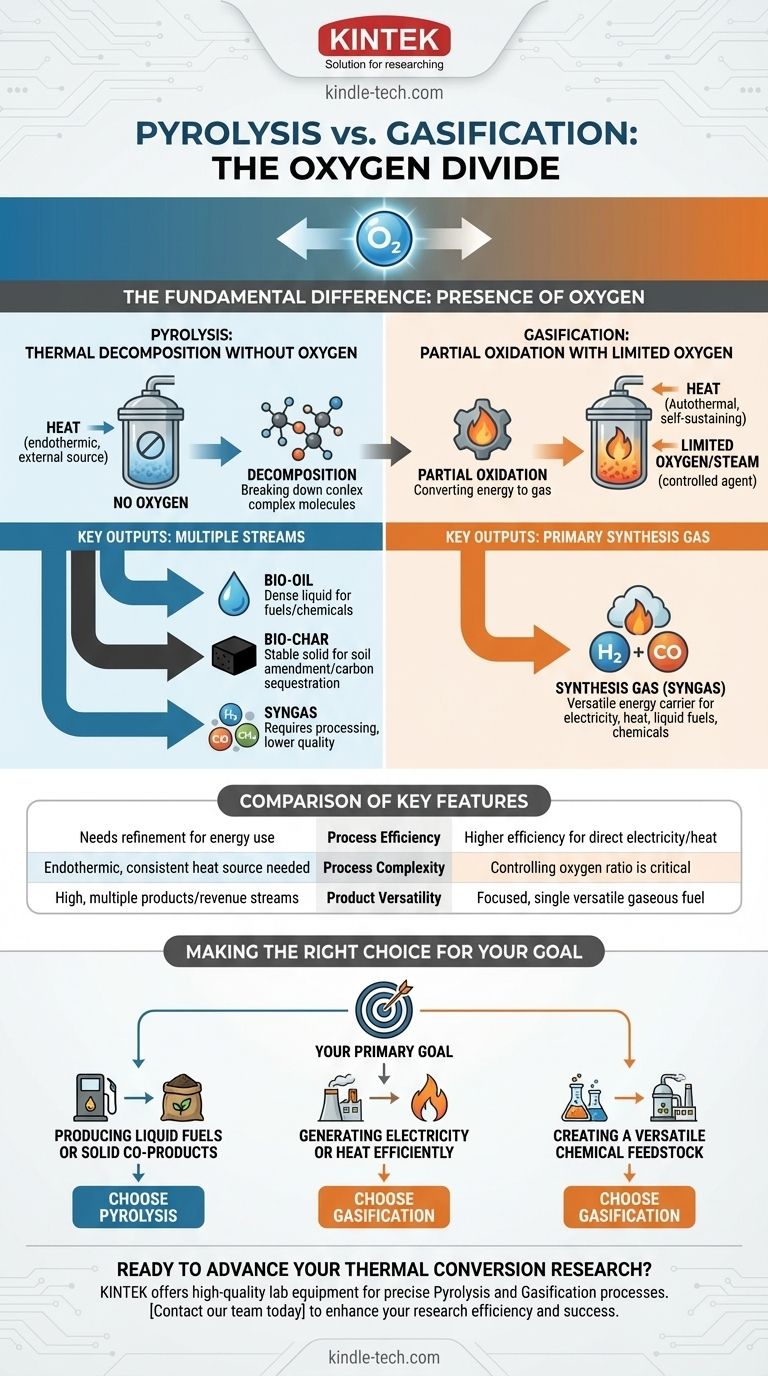
The fundamental difference between pyrolysis and gasification lies in the presence of oxygen. Pyrolysis is the thermal decomposition of organic material in a completely oxygen-free environment. In contrast, gasification uses a controlled, limited amount of oxygen or steam. This single distinction dictates the entire chemical pathway, determining the final products and their most effective applications.
Pyrolysis is a decomposition process, breaking down biomass in the absence of oxygen to create a mix of solid char, liquid bio-oil, and gas. Gasification is a conversion process, using limited oxygen to transform the majority of the biomass into a combustible synthesis gas (syngas).

The Core Differentiator: The Role of Oxygen
The presence or absence of oxygen is not a minor detail; it is the defining variable that separates these two powerful thermal conversion technologies.
Pyrolysis: Thermal Decomposition Without Oxygen
Pyrolysis is purely a thermal breakdown process. By heating organic material (like biomass) to high temperatures in an inert atmosphere, complex hydrocarbon molecules are broken apart into smaller, simpler ones.
Because there is no oxygen, no combustion occurs. This makes the process primarily endothermic, meaning it requires a continuous external heat source to drive the reaction. The goal is to "crack" the material into valuable chemical building blocks.
Gasification: Partial Oxidation with Limited Oxygen
Gasification intentionally introduces a restricted amount of an oxidizing agent (air, oxygen, and/or steam). This is not enough oxygen for complete combustion but just enough to cause partial oxidation.
This partial oxidation is exothermic, releasing energy that helps drive the gasification process, making it more thermally self-sufficient than pyrolysis. The goal is not to break the material down into its components, but to convert its chemical energy into a gaseous fuel.
A Comparison of Key Outputs
The different chemical environments of pyrolysis and gasification lead to distinctly different product slates, each with its own market and use case.
Pyrolysis Products: Bio-oil, Bio-char, and Gas
Pyrolysis creates three primary product streams:
- Bio-oil: A dense, acidic liquid often called "pyrolysis oil." It can be upgraded into transportation fuels or used to produce specialty chemicals.
- Bio-char: A stable, carbon-rich solid similar to charcoal. It is highly valuable as a soil amendment to improve fertility and sequester carbon.
- Syngas: A mixture of gases, including hydrogen and carbon monoxide, but also other hydrocarbons. This gas often requires an additional processing step, like catalytic reforming, to be used as a clean fuel.
Gasification Products: Primarily Synthesis Gas (Syngas)
Gasification is designed to maximize the production of one primary output: synthesis gas, or syngas.
This gas consists almost entirely of hydrogen (H2) and carbon monoxide (CO). Syngas is an incredibly versatile energy carrier, ready for immediate use in generating electricity and heat, or as a clean feedstock for producing liquid fuels and chemicals.
Understanding the Trade-offs
Choosing between these technologies requires an objective understanding of their operational demands and efficiencies.
Process Efficiency and Energy Output
Gasification is generally considered more efficient for the direct production of electricity and heat. It converts the bulk of the feedstock's energy into a combustible gas that can be used immediately in a generator or turbine.
The products from pyrolysis, particularly bio-oil and bio-char, retain a high energy content. However, they often require transportation, storage, and further refinement before that energy can be utilized, which can impact the overall system efficiency.
Process Complexity and Control
Pyrolysis is an endothermic process that requires a reliable and consistent external heat source.
Gasification's main complexity lies in precisely controlling the ratio of oxygen (or steam) to the feedstock. Too little oxygen and the process resembles pyrolysis; too much and it shifts toward complete combustion, reducing the quality of the syngas.
Product Versatility
Pyrolysis offers greater product diversity. The ability to create a valuable liquid (bio-oil) and a solid (bio-char) simultaneously can create multiple revenue streams.
Gasification is more focused, excelling at a single task: converting a solid feedstock into a clean, uniform gaseous fuel.
Making the Right Choice for Your Goal
Your final decision must be driven by your intended outcome. The technology is a tool, and you must select the right tool for the job.
- If your primary focus is producing liquid fuels or valuable solid co-products: Pyrolysis is the superior choice, as it yields bio-oil for transport and bio-char for soil amendment.
- If your primary focus is generating electricity or heat efficiently: Gasification is generally more direct, as it converts the bulk of the material into a combustible syngas ready for immediate use.
- If your primary focus is creating a versatile chemical feedstock: Gasification is often preferred, as its clean H2 and CO-rich syngas is a direct precursor for many industrial chemical syntheses.
Ultimately, selecting the right technology depends on whether your goal is to deconstruct biomass into valuable components or to convert its energy into a versatile gaseous fuel.
Summary Table:
| Feature | Pyrolysis | Gasification |
|---|---|---|
| Oxygen Environment | Completely absent (inert) | Limited, controlled amount |
| Primary Process | Thermal decomposition | Partial oxidation |
| Main Product(s) | Bio-oil, Bio-char, Syngas | Synthesis Gas (Syngas: H2 + CO) |
| Energy Requirement | Endothermic (needs external heat) | Autothermal (self-sustaining) |
| Best For | Liquid fuels, solid co-products | Electricity, heat, chemical feedstock |
Ready to select the right thermal conversion technology for your laboratory or project?
KINTEK specializes in high-quality lab equipment and consumables for advanced energy and material research. Whether you're developing pyrolysis processes for bio-oil and bio-char or optimizing gasification for syngas production, our experts can help you choose the right tools to achieve precise, reliable results.
Contact our team today to discuss your specific needs and discover how KINTEK's solutions can enhance your research efficiency and success.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















