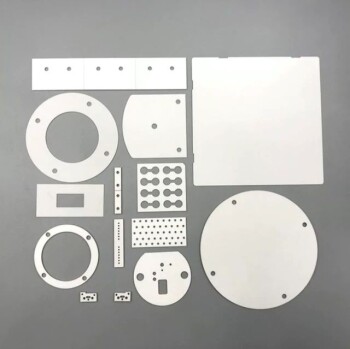
In principle, high-purity silica glass is one of the strongest materials known in terms of compressive strength. Under ideal, uniform compressive loading, a flawless piece of fused silica can withstand pressure exceeding 1.1 Gigapascals (GPa), or over 160,000 pounds per square inch (psi). However, this theoretical number is almost irrelevant in practice, as the usable strength of any glass component is dictated almost entirely by its tensile strength and the presence of microscopic surface flaws.
The critical takeaway is that the effective pressure a glass component can withstand is not an intrinsic material property. Instead, it is a system property defined by the size of its largest surface flaw, the type of loading (compressive vs. tensile), and the geometry of the component.

Compressive vs. Tensile Strength: The Two Faces of Glass
To understand the limits of glass, you must first distinguish between the two ways it handles force. This distinction is the single most important factor in its design and application.
Compressive Strength: An Atomic Fortress
Under compressive strength, atoms are pushed closer together. The amorphous, yet tightly bonded, atomic structure of silica glass excels at resisting this, distributing the force evenly across its powerful silicon-oxygen bonds.
This is why its theoretical compressive strength is so high, rivaling that of many metals. It is exceptionally difficult to crush a perfect piece of glass.
Tensile Strength: The Achilles' Heel
Tensile strength is the ability to resist being pulled apart. Here, glass is notoriously weak. Its practical tensile strength is orders of magnitude lower than its compressive strength, typically ranging from 30 to 60 MPa (4,000 to 9,000 psi).
The reason for this dramatic difference lies not in the atomic bonds themselves, but in the unavoidable imperfections on the surface of the material.
The Decisive Role of Surface Flaws
The practical strength of glass is a direct consequence of a principle known as Griffith's theory of fracture, which explains that failure almost always originates at a pre-existing flaw.
Micro-Cracks as Stress Concentrators
Every real-world piece of glass has microscopic scratches, pits, and cracks on its surface from manufacturing, handling, and environmental exposure. These are often called "Griffith flaws."
When a tensile force is applied, the stress becomes highly concentrated at the tip of the sharpest and deepest of these flaws. The force that would be distributed over a wide area is instead focused on a single, microscopic point.
How Failure Occurs
This stress concentration at the crack tip can easily exceed the material's local atomic bond strength, even when the overall applied force is low.
Once the bond breaks at that one point, the crack begins to propagate rapidly—often at nearly the speed of sound—resulting in catastrophic, brittle failure. This is why glass breaks suddenly and without warning.
Understanding the Trade-offs and Practical Limits
Simply knowing a material's strength value is insufficient for design. You must account for the factors that govern its performance in a real-world system.
Theoretical vs. Practical Strength
Never design a glass component based on its theoretical compressive strength. The effective strength is always limited by its much lower tensile strength and the presence of flaws. A safety factor of 10x or more is common in critical applications.
The Danger of Point Loads
A uniform, hydrostatic pressure (like deep-sea submersion) is the ideal compressive load. In contrast, a point load (e.g., a bolt head tightening directly on the glass surface) will create immense localized tensile stresses around the contact point, leading to rapid failure. Gaskets and proper mounting are essential to distribute loads.
Geometry and Edge Effects
The strength of a glass component is heavily influenced by its shape. Sharp corners, drill holes, and rough-cut edges are all significant stress concentrators. Polished, beveled, or "fire-polished" edges dramatically increase the strength and reliability of a glass part by removing the largest surface flaws.
Material Purity and Type
Not all glass is the same. The pressure it can handle varies significantly by its composition.
- Fused Silica: The purest form of silica glass (SiO₂). It has the highest strength, best thermal stability, and best optical transmission, but it is also the most expensive.
- Borosilicate Glass (e.g., Pyrex®, DURAN®): Contains boron trioxide, which gives it excellent thermal shock resistance and good chemical resistance. Its mechanical strength is lower than fused silica but higher than standard soda-lime glass.
- Soda-Lime Glass: The most common and least expensive type of glass, used for windows and bottles. It has the lowest mechanical strength and thermal resistance of the three.
Making the Right Choice for Your Goal
Your choice of material and design approach depends entirely on your application's primary requirement.
- If your primary focus is maximum pressure resistance and reliability: Use high-purity fused silica, ensure all surfaces and edges are highly polished, and design the system to keep the glass under uniform compression wherever possible.
- If your primary focus is balancing performance with thermal resistance: Borosilicate glass is an excellent, well-rounded choice suitable for laboratory equipment and industrial sight glasses where temperature and chemicals are a concern.
- If your primary focus is cost-effectiveness for a non-critical application: Soda-lime glass can be used, but you must design with a very large safety margin and understand its significantly lower performance limits.
By shifting your focus from a material's theoretical limit to the engineering context of its flaws and loading conditions, you can design systems that leverage the unique properties of glass safely and effectively.
Summary Table:
| Key Factor | Impact on Pressure Resistance |
|---|---|
| Compressive Strength (Theoretical) | >1.1 GPa (160,000 psi) - Very high, but rarely the limiting factor. |
| Tensile Strength (Practical) | 30-60 MPa (4,000-9,000 psi) - The true limit for most applications. |
| Surface Flaws (Scratches, Cracks) | Drastically reduce usable strength by concentrating stress. |
| Glass Type | Fused Silica (strongest) > Borosilicate > Soda-Lime (weakest). |
| Load Type | Uniform compression (good) vs. Point loads or bending (bad). |
Need a Reliable Glass Component for Your High-Pressure Application?
Designing with glass requires expert knowledge to navigate the critical gap between theoretical strength and real-world performance. KINTEK specializes in high-performance lab equipment and consumables, including custom glass components made from fused silica and borosilicate for demanding environments.
We help you:
- Select the right glass type (Fused Silica, Borosilicate) for your pressure, thermal, and chemical requirements.
- Optimize design and finishing (e.g., polished edges) to maximize strength and longevity.
- Ensure safe and reliable integration into your laboratory systems.
Don't leave your project's success to chance. Contact our experts today for a consultation on your specific needs!
Visual Guide
Related Products
- High Temperature Resistant Optical Quartz Glass Sheet
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Engineering Advanced Fine Ceramics Head Tweezers with Pointed Elbow Zirconia Ceramic Tip
- High-Purity Titanium Foil and Sheet for Industrial Applications
- High Temperature Wear-Resistant Alumina Al2O3 Plate for Engineering Advanced Fine Ceramics
People Also Ask
- What is the high temperature of quartz? Key Thresholds for Crystalline vs. Fused Silica
- Does quartz have a high melting point? Discover Its Superior High-Temperature Performance
- What are the thermal properties of quartz? Unlocking Extreme Temperature Stability for Your Lab
- How does quartz differ from glass? A Guide to Material Selection for Performance
- What is the temperature range of quartz glass? Master Its Thermal Limits for Demanding Applications