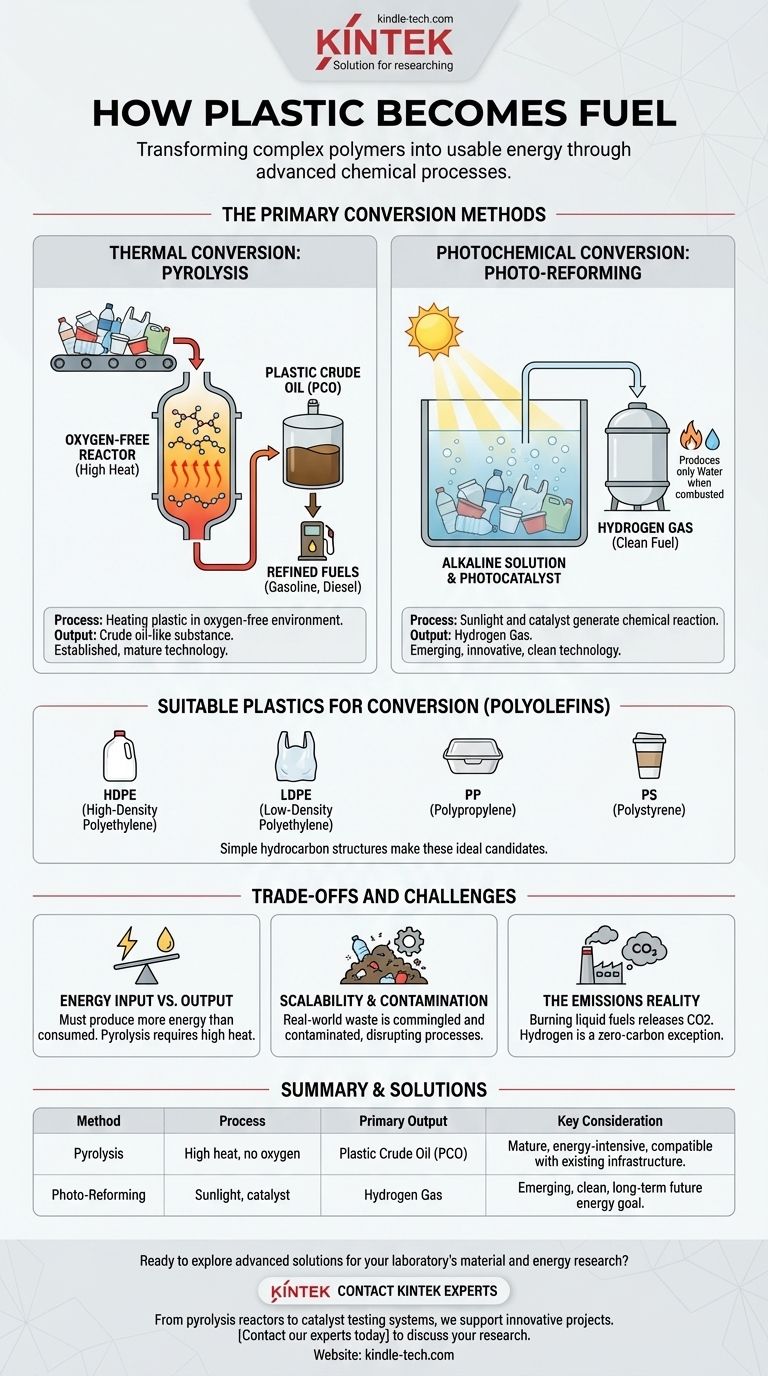
At its core, plastic can be converted into usable fuel through advanced chemical processes that break down its complex polymer structures. The two primary methods are pyrolysis, which uses high heat in an oxygen-free environment to create a crude oil-like substance, and photo-reforming, an emerging technique that uses sunlight and a catalyst to generate hydrogen gas directly from plastic waste.
While turning waste plastic into fuel presents a compelling solution to pollution, its true value depends entirely on the specific conversion technology, the type of plastic being processed, and the overall energy efficiency of the system.

The Primary Conversion Methods Explained
To understand how plastic becomes fuel, we must look at how its fundamental chemical structure—long chains of hydrocarbons—is broken down and repurposed.
Thermal Conversion: Pyrolysis
Pyrolysis is the most established method for creating liquid fuel from plastic. The process involves heating plastic waste to high temperatures in a reactor vessel that contains no oxygen.
This intense, oxygen-free heat causes the long polymer chains in the plastic to crack and break down into smaller, simpler hydrocarbon molecules.
The result is a substance called plastic crude oil (PCO). This oil can then be refined through distillation, much like traditional crude oil, to produce familiar fuels like gasoline and diesel.
Photochemical Conversion: Photo-reforming
A more recent and innovative approach is photo-reforming. This process uses light as its primary energy source.
Researchers have shown that by adding a light-absorbing material, known as a photocatalyst, to plastic submerged in an alkaline solution, sunlight can be used to power a chemical reaction.
This reaction breaks down the plastic and releases hydrogen gas. This is significant because hydrogen is a clean fuel that produces only water when combusted.
Which Plastics Are Suitable for Conversion?
Not all plastics are created equal. The chemical makeup of the plastic feedstock is critical to the efficiency and output of the fuel conversion process.
The Role of Polyolefins
The most common and effective plastics for fuel conversion are polyolefins. This category of plastics has a simple hydrocarbon structure (composed only of hydrogen and carbon) that closely resembles the molecules found in fossil fuels.
Common Examples
Plastics that are prime candidates for conversion include those that are often difficult to recycle through traditional means.
- High-Density Polyethylene (HDPE): Found in milk jugs, detergent bottles, and plastic bags.
- Low-Density Polyethylene (LDPE): Used for plastic bags and films.
- Polypropylene (PP): Used in containers, car parts, and carpets.
- Polystyrene (PS): Found in disposable cups and packaging materials.
Understanding the Trade-offs and Challenges
While promising, converting plastic to fuel is not a silver bullet. An objective analysis requires acknowledging the potential downsides and practical hurdles.
Energy Input vs. Energy Output
The most critical question for any energy production method is its net energy balance. For pyrolysis, a significant amount of energy is required to heat the plastic to the necessary temperatures. A successful operation must produce fuel with a higher energy value than the energy it consumed to create it.
Scalability and Contamination
Laboratory success does not always translate to industrial scale. Real-world plastic waste is a messy, commingled stream of different plastic types, often contaminated with food residue, paper, and other materials. These contaminants can disrupt the chemical process and reduce the quality of the final fuel.
The Emissions Reality
Claims that the process has no harmful emissions must be carefully qualified. While the conversion process itself can be contained, the end use of the fuel matters. Burning liquid fuels like diesel or gasoline derived from plastic will still release CO2 and other pollutants into the atmosphere. The notable exception is hydrogen, which is a zero-carbon fuel at the point of use.
Making the Right Choice for Your Goal
The best plastic-to-fuel strategy depends on your primary objective, whether it's immediate waste management or long-term clean energy production.
- If your primary focus is leveraging existing infrastructure: Pyrolysis is the more mature technology, producing liquid fuels that are compatible with current engines and distribution networks.
- If your primary focus is a future clean energy system: Photo-reforming to create hydrogen is a powerful long-term goal, as it produces a truly clean-burning fuel.
- If your primary focus is immediate waste reduction: Both methods provide a valuable alternative to landfilling for hard-to-recycle plastics, turning a liability into a potential asset.
Ultimately, using plastic as a feedstock for fuel transforms a persistent waste problem into a potential energy resource, though its practical application requires careful technical and environmental evaluation.
Summary Table:
| Method | Process | Primary Output | Key Consideration |
|---|---|---|---|
| Pyrolysis | High heat without oxygen | Plastic Crude Oil (PCO) | Mature technology, but requires significant energy input |
| Photo-Reforming | Sunlight with a catalyst | Hydrogen Gas | Emerging clean fuel technology, but less developed |
Ready to explore advanced solutions for your laboratory's material and energy research?
At KINTEK, we specialize in providing high-quality lab equipment and consumables to support innovative projects—from pyrolysis reactors to catalyst testing systems. Our expertise can help you accurately evaluate and develop plastic-to-fuel conversion processes.
Contact our experts today to discuss how our solutions can power your research and contribute to a sustainable future.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond for Thermal Management Applications
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- High Performance Laboratory Freeze Dryer
People Also Ask
- How does a rotary vacuum evaporator work? A Guide to Gentle, Efficient Solvent Removal
- What is the problem in heat treatment process? The High Cost of Preventing Surface Defects
- What is the function of a forced air drying oven in the regeneration cycle of dolomite catalysts? Optimize Your Lab Results
- What is the cost for biomass energy? Understanding the $0.06-$0.15/kWh Range and Key Drivers
- What is a furnace used in the lab? Your Guide to High-Temperature Precision
- What are the components of a pyrolysis reactor? A Guide to Core Parts & Designs
- What is sputter coating? A High-Performance Thin Film Deposition Process
- What varieties of high-temperature furnaces are available? Find the Perfect Lab Furnace for Your Thermal Research



















