In the context of thin-film deposition, both sputtering and evaporation are capable of producing high-purity films. However, sputtering is generally favored for applications where film quality, density, and adhesion are as critical as purity. The final purity achieved by either method is less about the technique itself and more about the quality of the source material and the control over the process environment.
The choice between sputtering and evaporation is not a simple question of which is "purer." It is a strategic decision that requires balancing the need for purity against other critical film properties like density, adhesion, and deposition speed.

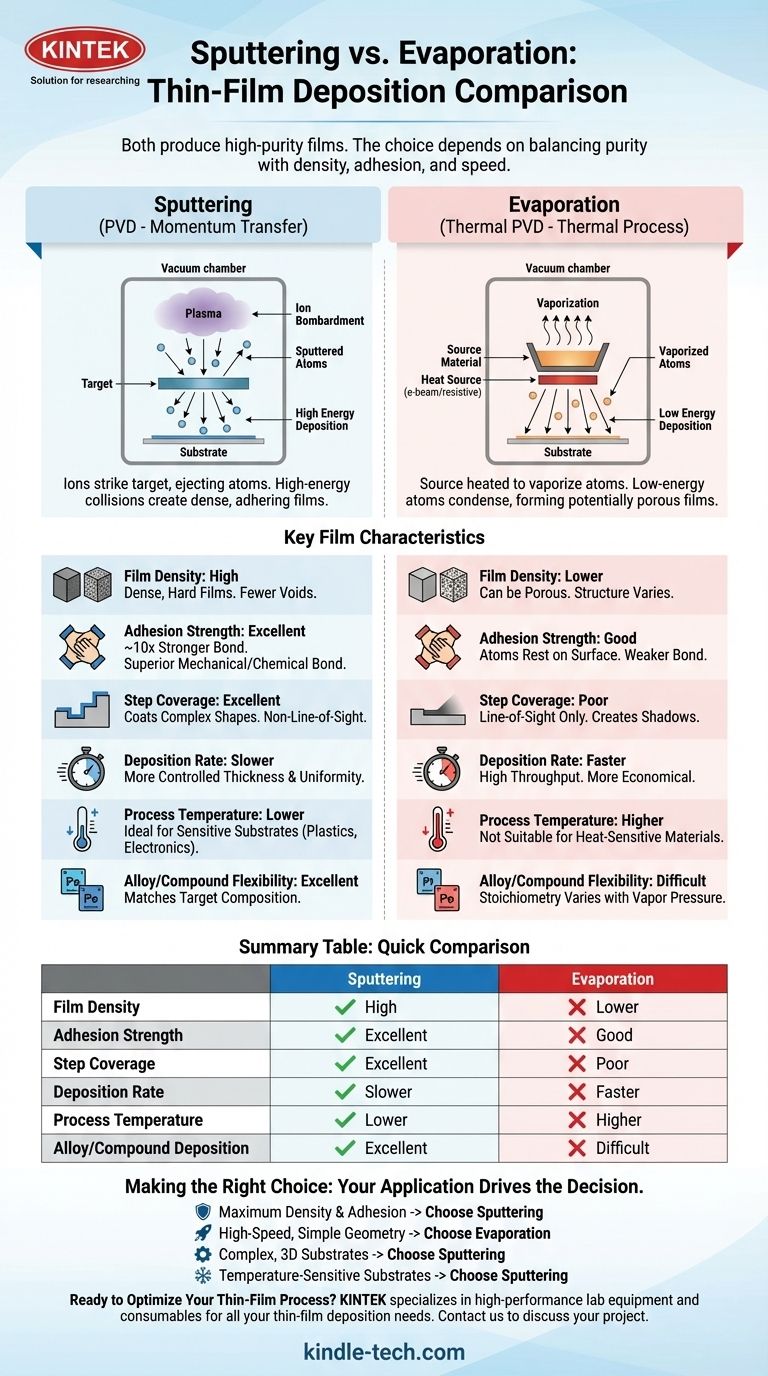
How the Deposition Method Defines Film Quality
To understand the differences in outcome, you must first understand the fundamental difference in how each process works. Both are forms of Physical Vapor Deposition (PVD), but they arrive at the same goal—depositing a thin film—via entirely different physical mechanisms.
Evaporation: A Thermal Process
Evaporation, including e-beam evaporation, is essentially a process of boiling a material in a vacuum. A high-energy electron beam or a resistive heater heats the source material in a crucible until its atoms vaporize.
These vaporized atoms then travel in a straight line through the vacuum chamber until they condense on the cooler substrate, forming a film. Think of it like steam from a kettle condensing on a cold mirror.
Sputtering: A Momentum Transfer Process
Sputtering does not involve melting or boiling. Instead, an inert gas like argon is introduced into the chamber and ionized to create a plasma. A strong electric field accelerates these ions, causing them to collide with the source material (the "target") with great force.
These high-energy collisions are like a microscopic sandblasting, knocking individual atoms or molecules off the target. These ejected particles then travel and deposit onto the substrate, building the film layer by layer.
Comparing Key Film Characteristics
The differences in these physical mechanisms directly lead to different film properties. Purity is only one part of the story.
Purity and Density
While both methods can use exceptionally pure source materials, sputtered films are almost always denser and harder. The high kinetic energy of the sputtered atoms effectively "hammers" them into place, creating a more compact film with fewer voids.
In evaporation, atoms land with much less energy, which can lead to a more porous film structure. Regarding purity, any contamination in the vacuum chamber can compromise an evaporated film. In sputtering, the purity of the process gas (argon) is an additional variable that must be controlled.
Adhesion Strength
Sputtering provides significantly better film adhesion. The reference materials note it can be ten times greater than that of evaporated films.
This is because the high-energy sputtered particles embed themselves slightly into the substrate's surface, creating a superior mechanical and chemical bond. Evaporated atoms, with their low energy, simply rest on the surface.
Step Coverage and Uniformity
Sputtering provides far better coverage on complex, non-flat surfaces. Because the sputtered atoms are knocked off the target at various angles and can scatter off gas molecules, they coat the sides of features and not just the top.
Evaporation is a "line-of-sight" process. Any part of the substrate that is not in the direct path of the vapor source will receive little to no coating, creating "shadows."
Understanding the Trade-offs
Neither method is universally superior; the choice involves clear engineering trade-offs.
Deposition Rate vs. Control
Evaporation is typically a much faster deposition process. For applications where high throughput is critical and film structure is less demanding, evaporation is often the more economical choice.
Sputtering is a slower, more deliberate process. This lower deposition rate, however, allows for extremely precise control over film thickness and uniformity across the substrate.
Process Temperature
Sputtering is fundamentally a lower-temperature process. This makes it ideal for coating heat-sensitive materials like plastics or pre-existing electronic components that could be damaged by the intense heat of e-beam evaporation.
Material Compatibility
Sputtering offers greater flexibility for depositing alloys or compounds. Since the material is knocked off the target mechanically, the composition of the film is very close to the composition of the target.
In evaporation, materials with different vapor pressures will evaporate at different rates, making it very difficult to maintain the correct stoichiometry for an alloy.
Making the Right Choice for Your Application
Your decision should be driven by the most important characteristic of your final product.
- If your primary focus is maximum film density and superior adhesion: Sputtering is the superior choice, creating a robust and durable film that is tightly bonded to the substrate.
- If your primary focus is high-speed deposition for simple geometries: E-beam or thermal evaporation offers unmatched throughput and is more cost-effective for high-volume production.
- If your primary focus is coating complex, three-dimensional substrates: Sputtering's ability to coat non-line-of-sight surfaces provides far better and more uniform coverage.
- If your primary focus is working with temperature-sensitive substrates: Sputtering's lower process temperature is a significant advantage that prevents damage to the underlying material.
Ultimately, selecting the right deposition technique requires a clear understanding of your application's specific technical and economic requirements.
Summary Table:
| Characteristic | Sputtering | Evaporation |
|---|---|---|
| Film Density | High (Dense, hard films) | Lower (Can be porous) |
| Adhesion Strength | Excellent (10x stronger) | Good |
| Step Coverage | Excellent (Coats complex shapes) | Poor (Line-of-sight only) |
| Deposition Rate | Slower, more controlled | Faster, high throughput |
| Process Temperature | Lower (Ideal for sensitive substrates) | Higher |
| Alloy/Compound Deposition | Excellent (Matches target composition) | Difficult (Varies with vapor pressure) |
Ready to Optimize Your Thin-Film Process?
Choosing between sputtering and evaporation is critical for the performance of your final product. The right equipment ensures superior film quality, adhesion, and yield.
KINTEK specializes in high-performance lab equipment and consumables for all your thin-film deposition needs. Whether you require the robust, dense films of a sputtering system or the high-throughput capabilities of an evaporation system, our experts can help you select the perfect solution for your laboratory's specific requirements.
Contact us today using the form below to discuss your project and discover how KINTEK can enhance your research and production capabilities.
Visual Guide
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- CVD Diamond Cutting Tool Blanks for Precision Machining
People Also Ask
- What substrates are used for thin film deposition? Choosing the Right Foundation for Your Application
- What is the vacuum evaporation method? A Guide to Thin Film Deposition & Purification
- What are the disadvantages of thermal evaporation? Key Limitations in Purity, Density & Materials
- What type of deposition is resulted at high vacuum? Achieve Pure, High-Performance Thin Films with PVD
- How is the thickness of a deposited film measured? Master Optical Interference Techniques
- What is the use of physical vapor deposition? Enhance Durability, Performance, and Purity
- Why are high-temperature porcelain boats used for biochar in a tube furnace? Ensure Sample Purity and Thermal Stability
- What are the applications of electron beams? From Nanoscale Imaging to Industrial Manufacturing



















