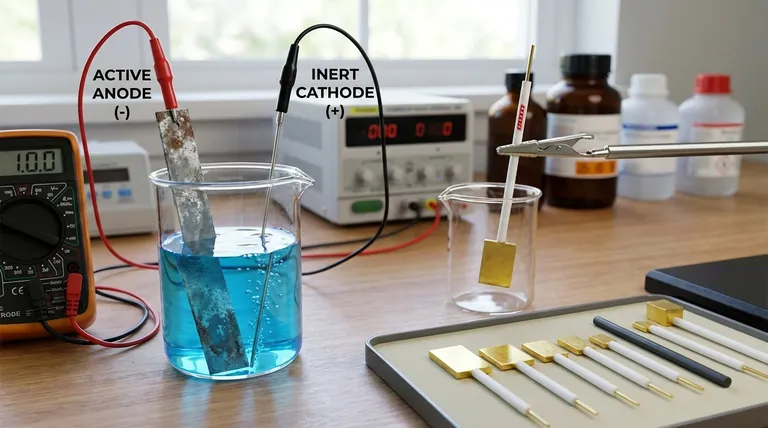
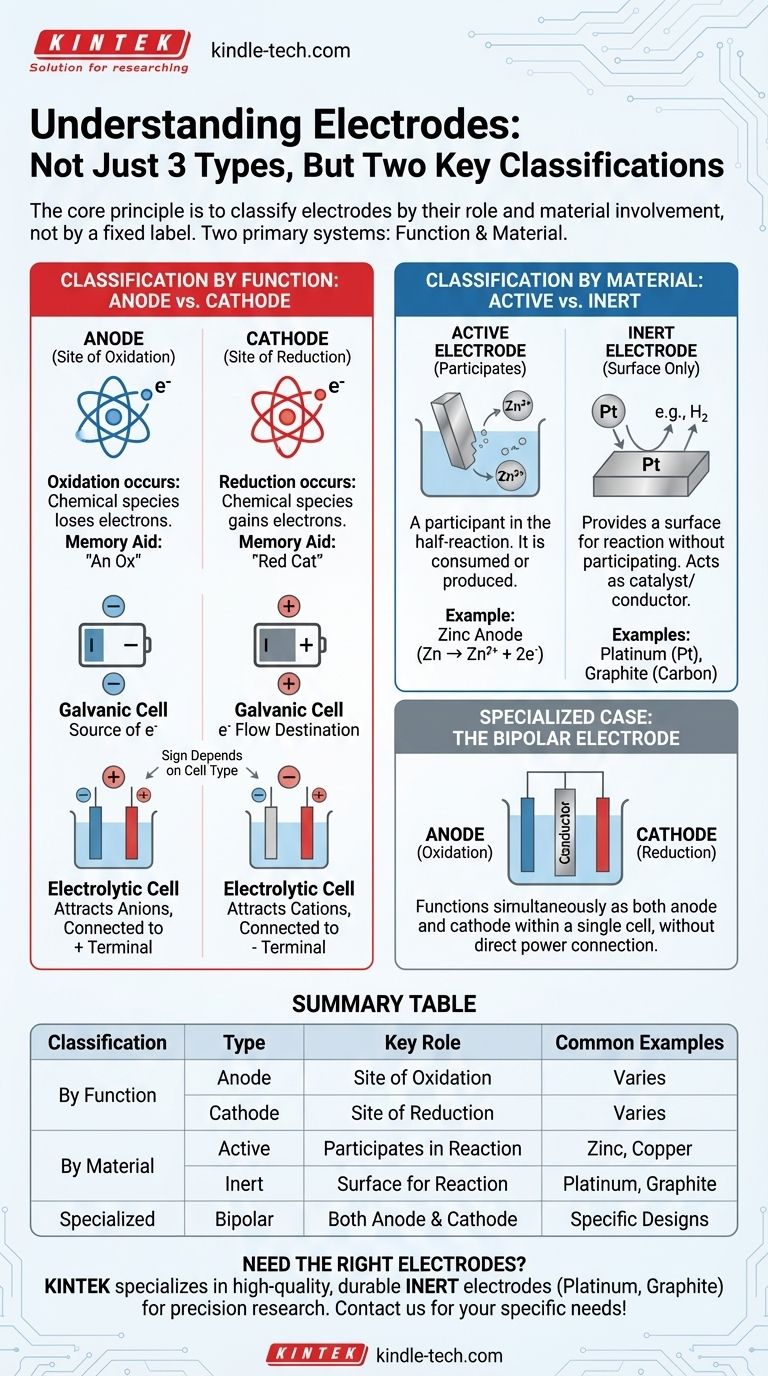
In electrochemistry, electrodes are best understood not as three distinct types, but through two primary classification systems. The most fundamental system defines an electrode by its function: either an anode, where oxidation occurs, or a cathode, where reduction occurs. A second, equally important system classifies them by their material composition and role in the reaction: either active (participating in the reaction) or inert (providing a surface for reaction).
The core principle is to classify electrodes by their role, not by a fixed label. The two most critical classifications are based on function (Anode vs. Cathode) and material involvement (Active vs. Inert). Understanding these two pairs is the key to analyzing any electrochemical cell.
Classification by Function: Anode vs. Cathode
The most fundamental way to define an electrode is by the chemical process that happens on its surface. This role can change depending on the flow of electricity.
The Anode: Site of Oxidation
The anode is defined as the electrode where oxidation takes place. This is a process where a chemical species loses electrons.
A simple memory aid is "An Ox" (Anode is Oxidation).
The Cathode: Site of Reduction
The cathode is the electrode where reduction takes place. This is a process where a chemical species gains electrons.
A corresponding memory aid is "Red Cat" (Reduction at the Cathode).
The Critical Distinction: Sign vs. Function
A common point of confusion is whether the anode and cathode are positive or negative. This depends entirely on the type of electrochemical cell.
In a Galvanic Cell (like a Battery)
A galvanic cell produces electricity from a spontaneous reaction.
- The Anode is the Negative (-) terminal. It is the source of electrons from the oxidation reaction.
- The Cathode is the Positive (+) terminal. It is where electrons flow to cause the reduction reaction.
In an Electrolytic Cell (like for Plating)
An electrolytic cell uses external electricity to drive a non-spontaneous reaction.
- The Anode is the Positive (+) terminal. It is connected to the positive end of the power source, attracting anions to be oxidized.
- The Cathode is the Negative (-) terminal. It is connected to the negative end of the power source, attracting cations to be reduced.
Classification by Material: Active vs. Inert
The second major classification describes whether the electrode material itself is part of the chemical reaction.
Active Electrodes
An active electrode (or reactive electrode) is a participant in the half-reaction. It is made of a material that is either oxidized or is the product of a reduction.
For example, in a zinc-copper battery, the zinc anode physically dissolves (oxidizes) into zinc ions (Zn → Zn²⁺ + 2e⁻). The electrode itself is consumed.
Inert Electrodes
An inert electrode simply provides a surface for oxidation or reduction to occur without participating in the reaction itself. It acts as a catalyst and an electrical conductor.
Common inert electrodes include platinum (Pt) and graphite (carbon). They are used when the species being oxidized or reduced are ions or gases in the solution.
Specialized Case: The Bipolar Electrode
A bipolar electrode is a specialized conductor placed in an electrolyte solution that is not directly connected to the power source.
It functions simultaneously as an anode on the side facing the main cathode and as a cathode on the side facing the main anode, enabling a series of reactions within a single cell.
Making the Right Decision
Your understanding of electrodes should guide how you analyze an electrochemical system. The "type" of electrode is defined by its context within the cell.
- If you are analyzing a battery (galvanic cell): Identify the site of oxidation as the negative anode and the site of reduction as the positive cathode.
- If you are analyzing an electrolytic process (e.g., electroplating): Identify the electrode connected to the positive terminal as the anode (oxidation) and the one connected to the negative terminal as the cathode (reduction).
- When designing an experiment: You must decide whether you need an active electrode that participates in the reaction or an inert electrode to simply facilitate a reaction between species already in the solution.
By focusing on function and material involvement, you can accurately describe and predict the behavior of any electrode in any electrochemical system.
Summary Table:
| Classification | Type | Key Role | Common Examples |
|---|---|---|---|
| By Function | Anode | Site of Oxidation (loses electrons) | Varies by cell type |
| Cathode | Site of Reduction (gains electrons) | Varies by cell type | |
| By Material | Active | Participates in the reaction | Zinc, Copper |
| Inert | Provides surface for reaction (non-reactive) | Platinum, Graphite | |
| Specialized | Bipolar | Functions as both anode and cathode | Used in specific cell designs |
Need the right electrodes for your electrochemical application? KINTEK specializes in high-quality lab equipment and consumables, including durable inert electrodes made from platinum and graphite, designed for precision and reliability in your laboratory. Let our experts help you select the perfect components for your research or process. Contact us today to discuss your specific needs!
Visual Guide
Related Products
- Gold Electrochemical Sheet Electrode Gold Electrode
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What is the critical rule for using a gold plate electrode? Ensure Only the Gold Surface Contacts the Electrolyte
- What precautions should be taken to prevent mechanical damage to a gold plate electrode? Protect Your Data Integrity
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- What are the disadvantages of gold electrodes? Key Limitations for Your Lab Projects
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data



















