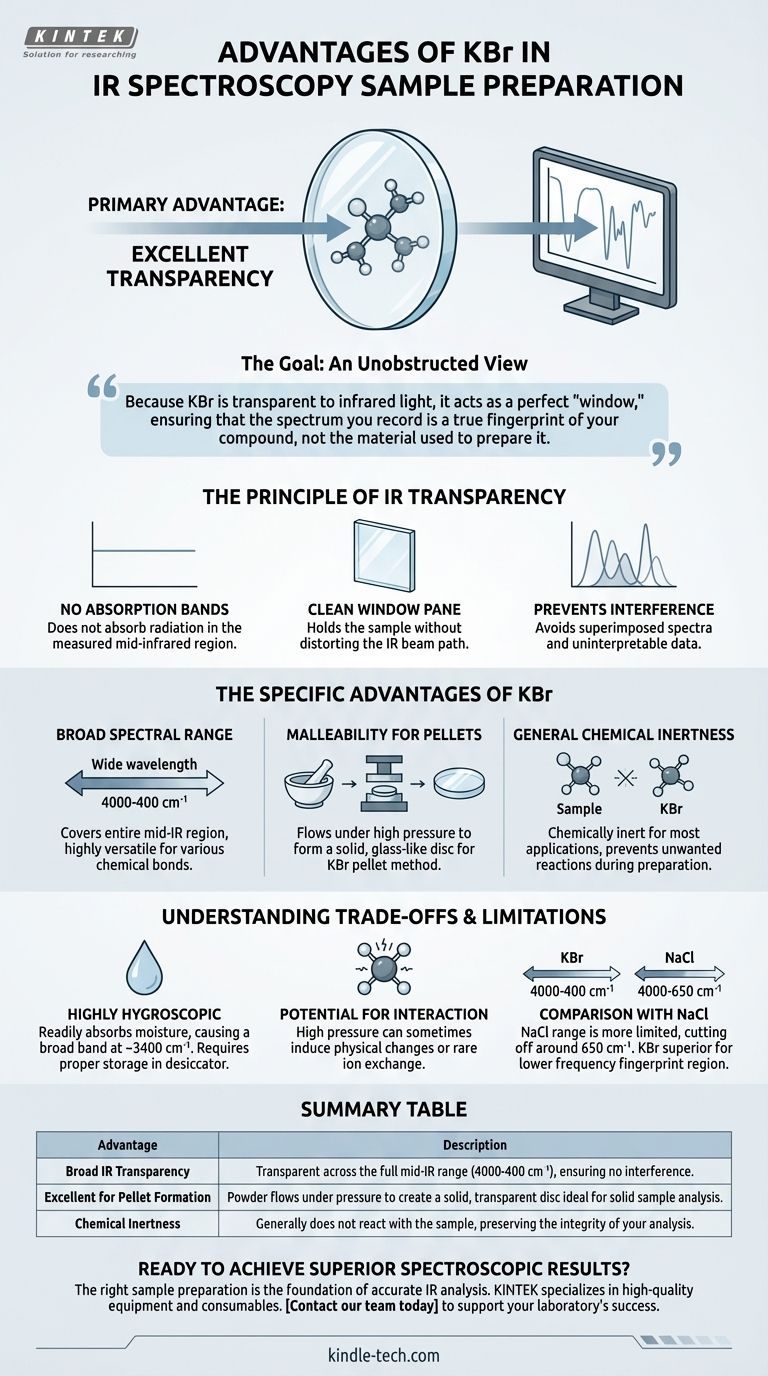
The primary advantage of using Potassium Bromide (KBr) in IR spectroscopy is its excellent transparency to infrared radiation across a very broad spectral range. This property is crucial because it ensures the material holding the sample does not itself absorb the IR light, which would interfere with the analysis. Using KBr allows the resulting spectrum to be a clean and accurate representation of the sample's molecular vibrations alone.
The choice of KBr is not arbitrary; it is a strategic decision to create a non-interfering environment for your sample. Because KBr is transparent to infrared light, it acts as a perfect "window," ensuring that the spectrum you record is a true fingerprint of your compound, not the material used to prepare it.

Why Sample Preparation is the Foundation of Good Spectra
The Principle of IR Transparency
In spectroscopy, a material is "transparent" if it does not absorb radiation in the wavelength range being measured. For a material to be useful as a sample holder or matrix in IR, it must not have its own vibrational absorption bands in the mid-infrared region where most organic and inorganic compounds are analyzed.
The Goal: An Unobstructed View
Think of the KBr as a perfectly clean window pane. Its purpose is to hold the sample in the path of the IR beam without distorting the "view." If the window pane were tinted or dirty (i.e., if it absorbed IR light), you couldn't get a clear picture of what's outside (the sample's spectrum).
Consequences of Poor Material Choice
Using a material that absorbs IR light will superimpose its own spectrum onto your sample's spectrum. This leads to confusing or uninterpretable data, where you cannot distinguish between the peaks from your sample and the peaks from the holder.
The Specific Advantages of KBr
Broad Spectral Range
KBr is transparent across a wide range, typically from 4000 cm⁻¹ down to 400 cm⁻¹. This covers the entire mid-IR region, making it a highly versatile and reliable choice for analyzing a vast array of different chemical bonds and functional groups.
Malleability for Pellet Formation
One of the most common solid sampling techniques is the KBr pellet method. KBr powder has the useful physical property of flowing under high pressure to form a solid, glass-like disc. By grinding a tiny amount of a solid sample with dry KBr and pressing it in a die, you can create a transparent pellet that is ideal for analysis.
General Chemical Inertness
For most applications, KBr is chemically inert and will not react with the sample. This prevents the formation of new, unexpected compounds during sample preparation that would compromise the integrity of the analysis.
Understanding the Trade-offs and Limitations
It is Highly Hygroscopic
The most significant disadvantage of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This absorbed water will produce a very broad absorption band in the IR spectrum (around 3400 cm⁻¹) which can obscure important N-H or O-H stretching vibrations from your actual sample. Proper storage in a desiccator and minimal exposure to air are critical.
Potential for Sample Interaction
While generally inert, the high pressure used to form a pellet can sometimes induce physical changes in the sample. In rare cases, certain compounds can undergo ion exchange with the bromide, altering the sample's chemical nature.
Comparison with Sodium Chloride (NaCl)
Sodium Chloride (NaCl) is another common salt used for IR windows, often called "salt plates." While also transparent, its useful range is more limited than KBr, typically cutting off around 650 cm⁻¹. KBr is the superior choice for analyzing the lower frequency "fingerprint" region of the spectrum.
Making the Right Choice for Your Analysis
Choosing the correct sample preparation technique is fundamental to success. Your decision should be guided by the nature of your sample and the information you need to obtain.
- If your primary focus is general-purpose analysis of a stable solid: The KBr pellet method is an excellent, cost-effective, and widely used standard that provides high-quality spectra.
- If your sample is sensitive to moisture or pressure: You should consider alternative techniques, such as Attenuated Total Reflectance (ATR) spectroscopy, which analyzes samples directly with minimal preparation.
- If you are working with a liquid or solution: You would use liquid cells made with KBr or NaCl windows, sandwiching a thin film of the liquid between two polished salt plates.
Ultimately, understanding the properties of your sample holder is the first step toward achieving reliable and accurate spectroscopic results.
Summary Table:
| Advantage | Description |
|---|---|
| Broad IR Transparency | Transparent across the full mid-IR range (4000-400 cm⁻¹), ensuring no interference. |
| Excellent for Pellet Formation | Powder flows under pressure to create a solid, transparent disc ideal for solid sample analysis. |
| Chemical Inertness | Generally does not react with the sample, preserving the integrity of your analysis. |
Ready to achieve superior spectroscopic results in your lab?
The right sample preparation is the foundation of accurate IR analysis. KINTEK specializes in providing high-quality laboratory equipment and consumables, including reliable materials for spectroscopy.
Let our experts help you select the perfect tools for your specific needs. Contact our team today to discuss how we can support your laboratory's success and ensure the integrity of your research.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Lab Infrared Press Mold
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
People Also Ask
- How do you make biomass pellets at home? A Step-by-Step Guide to DIY Fuel Production
- What are the preventive maintenance of hydraulic press machine? Maximize Uptime and Prevent Costly Failures
- How do you make an XRF sample? Choose the Right Prep Method for Accurate Results
- What role do hydraulic presses play in graphene transfer? Achieve Precise Bonding and Defect-Free Membranes
- How is a laboratory hydraulic press used to evaluate the stability of solid biopesticide formulations? Optimize Pellets
- What are the safety rules for a hydraulic press? Essential Protocols for Operator and Machine Safety
- What is the purpose of using a laboratory hydraulic press for pre-pressure? Enhance Sintering Precision & Density
- Why is a hydraulic press used for room temperature pressing in the manufacturing of diamond saw blades? Key Advantages



















