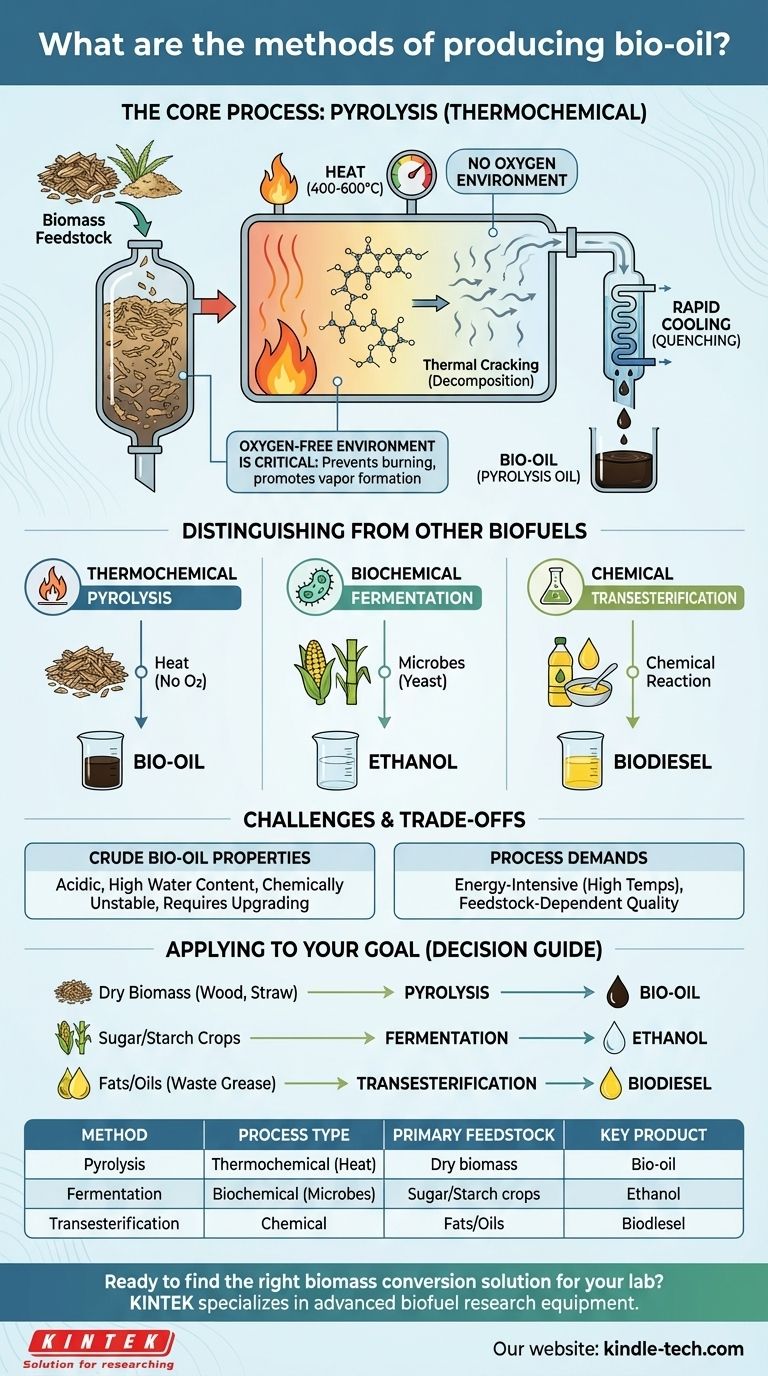
The principal method for producing bio-oil is a thermochemical process known as pyrolysis. This technique involves heating organic material, such as wood chips or agricultural waste, to high temperatures in an environment with little to no oxygen. This prevents the material from burning and instead causes it to thermally decompose into vapors, which are then rapidly cooled and condensed into a liquid: bio-oil.
At its core, bio-oil production is about converting bulky, solid biomass into a dense, liquid energy carrier. This is achieved through the thermal shock of pyrolysis, which breaks down complex organic matter into vapors that are then captured as a crude, renewable oil.

The Core Process: Understanding Pyrolysis
Pyrolysis is the central technology for converting a wide range of solid biomass into liquid bio-oil. The process is precise and depends on carefully controlling a few key variables.
How Pyrolysis Works
The process involves heating biomass to temperatures typically between 400-600°C. This is done in a reactor that has been purged of oxygen.
Instead of combusting (burning), the intense heat causes the long-chain molecules within the biomass to crack and break apart into smaller, volatile molecules.
The Critical Step: Rapid Cooling
These hot gases and vapors are then moved out of the reactor and cooled extremely quickly, a step known as quenching.
This rapid cooling freezes the chemical breakdown reactions and forces the vapors to condense into a liquid. This resulting dark, viscous liquid is what we call pyrolysis oil, or bio-oil.
The Importance of an Oxygen-Free Environment
The absence of oxygen is the defining characteristic of pyrolysis. If oxygen were present, the biomass would simply burn, producing ash, CO2, and water.
By eliminating oxygen, we ensure the biomass decomposes into the desired combustible gases and vapors that can be condensed into a liquid fuel.
Distinguishing Pyrolysis from Other Biofuel Processes
The term "biofuel" covers many different products made through distinct methods. It is critical not to confuse pyrolysis with the biochemical processes used to make other common biofuels.
Thermochemical vs. Biochemical
Pyrolysis is a thermochemical process; it uses heat to chemically change the biomass.
In contrast, fuels like ethanol and biodiesel are typically made via biochemical or other chemical pathways.
Fermentation for Ethanol
Ethanol is produced through fermentation, a biological process where microbes (like yeast) consume sugars from crops like corn or sugarcane and convert them into alcohol.
Transesterification for Biodiesel
Biodiesel is created through a chemical process called transesterification, which converts fats, greases, and vegetable oils into a diesel-like fuel. These methods are fundamentally different from the thermal decomposition of pyrolysis.
Understanding the Trade-offs and Challenges
While pyrolysis is a powerful conversion technology, it's important to recognize the nature of its product and the challenges involved.
The Nature of Crude Bio-oil
The resulting bio-oil is not a drop-in replacement for conventional crude oil. It is often acidic, contains significant amounts of water, and can be chemically unstable over time.
Because of these properties, bio-oil typically requires further upgrading or refining before it can be used in engines or traditional refineries.
Energy and Feedstock Demands
The process itself is energy-intensive, requiring high temperatures to be achieved and maintained. The net energy balance of the operation is a key factor in its viability.
Furthermore, the quality of the final bio-oil is highly dependent on the type and consistency of the biomass feedstock used.
Applying This to Your Goal
The right biomass conversion strategy depends entirely on your starting material and your desired end product.
- If your primary focus is converting dry, non-food biomass (like wood or straw) into a liquid intermediate for fuel or chemicals: Pyrolysis is the most direct and established thermochemical method.
- If your primary focus is producing fuel from sugar or starch-rich crops: Biochemical processes like fermentation to create ethanol are the appropriate pathway.
- If your primary focus is converting waste oils or purpose-grown oilseed crops into fuel: Chemical transesterification to produce biodiesel is the standard approach.
Understanding these distinct conversion pathways is the first step in effectively harnessing biomass as a renewable resource.
Summary Table:
| Method | Process Type | Primary Feedstock | Key Product |
|---|---|---|---|
| Pyrolysis | Thermochemical (Heat) | Dry biomass (wood, straw) | Bio-oil (Pyrolysis Oil) |
| Fermentation | Biochemical (Microbes) | Sugar/Starch crops (corn, sugarcane) | Ethanol |
| Transesterification | Chemical | Fats/Oils (vegetable oil, waste grease) | Biodiesel |
Ready to find the right biomass conversion solution for your lab? KINTEK specializes in lab equipment and consumables for advanced biofuel research, including pyrolysis reactors and analysis tools. Our experts can help you select the perfect setup to optimize your bio-oil production process. Contact our team today to discuss your specific needs and accelerate your renewable energy projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the advantages of spray pyrolysis? Achieve Cost-Effective, Scalable Thin Film Production
- In which furnace is calcination and roasting done? A Guide to Selecting the Right Thermal Processing Equipment
- What is the mechanism of pyrolysis of biomass materials? A Guide to Converting Biomass into Bio-Oil, Char, and Gas
- Is there a market for pyrolysis oil? A Guide to Opportunities and Challenges
- What is the pyrolysis method for biochar production? A Guide to Maximizing Carbon-Rich Char Yield
- Does pyrolysis use a lot of energy? Achieve Net Energy Positive Waste Conversion
- Why is pyrolysis sustainable? Unlocking a Circular Economy with Waste-to-Value Technology
- What is the operating temperature of pyrolysis? Master the Key to Biochar, Bio-oil, and Syngas Production



















