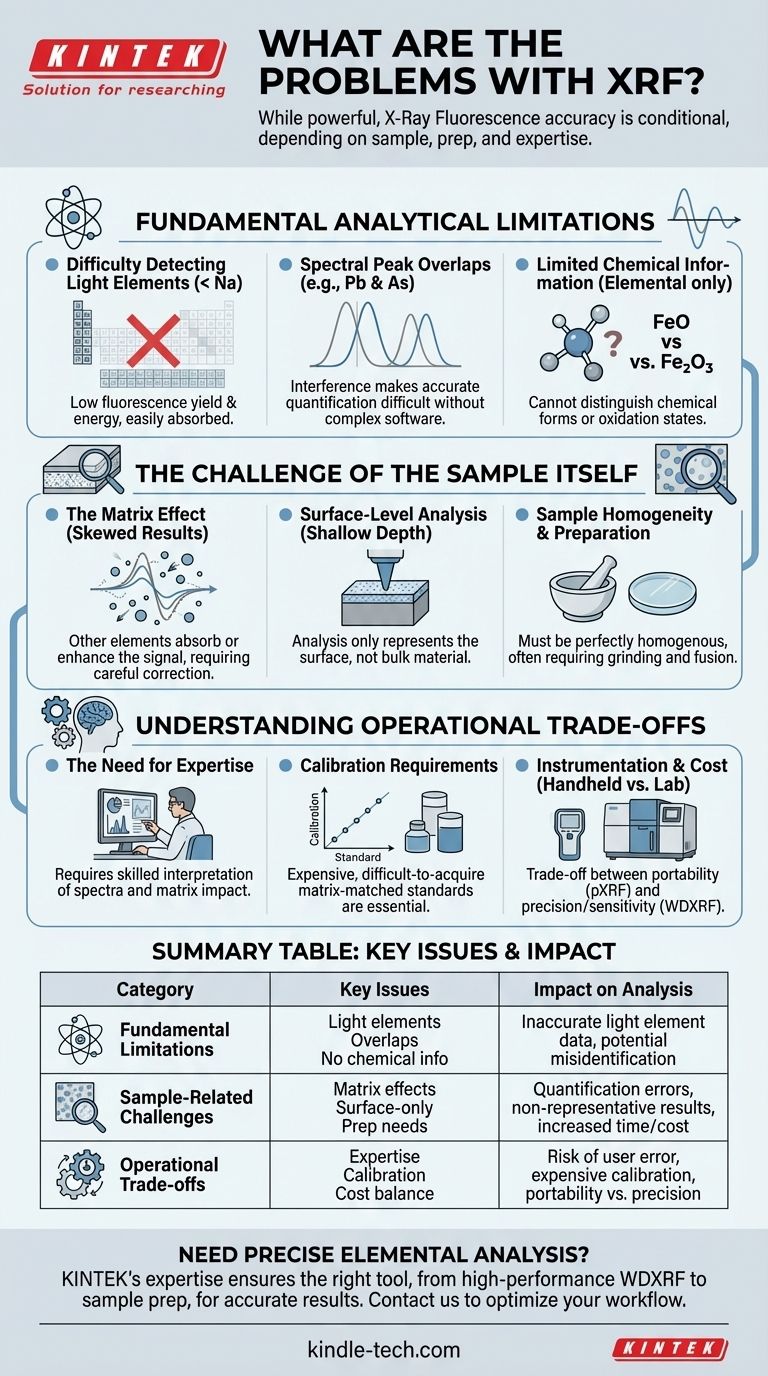
While a powerful and widely-used analytical tool, X-Ray Fluorescence (XRF) is not without significant limitations. Its core problems stem from fundamental physics, the nature of the sample being analyzed, and the practical requirements for obtaining accurate data. Understanding these constraints is critical to avoid misinterpreting results and to determine if XRF is the right tool for your specific application.
The central challenge with XRF is that its accuracy is not inherent; it is highly conditional. The reliability of your results depends entirely on the sample's composition (the "matrix"), proper sample preparation, and the operator's ability to navigate limitations like poor light-element detection and spectral overlaps.
Fundamental Analytical Limitations
The physics behind X-ray fluorescence imposes several key constraints on what the technique can and cannot measure effectively.
Difficulty Detecting Light Elements
XRF struggles to detect very light elements, typically those with an atomic number below 11 (Sodium). This is due to two primary reasons: first, light elements have a very low fluorescence yield, and second, the low-energy X-rays they do emit are easily absorbed by the air or the instrument's detector window before they can be measured.
Spectral Peak Overlaps
In complex samples containing many different elements, the characteristic X-ray emission lines of one element can overlap with the lines of another. For example, the L-alpha peak of Lead (Pb) and the K-alpha peak of Arsenic (As) are very close. This interference can make it difficult to accurately quantify either element without sophisticated software and expert analysis.
Limited Chemical Information
XRF is a technique for elemental analysis. It tells you what elements are present and in what concentration, but it provides little to no information about the chemical form or oxidation state. For example, XRF can measure the total amount of iron (Fe) in a sample, but it cannot distinguish between different iron oxides like FeO and Fe₂O₃.
The Challenge of the Sample Itself
An XRF instrument is only as good as the sample presented to it. The physical and chemical nature of your sample is often the largest source of error.
The Matrix Effect
The "matrix" refers to everything in the sample other than the specific element you are trying to measure. These other elements can absorb or enhance the X-ray signal of the target element, skewing the results. This matrix effect is one of the most significant challenges in quantitative XRF and requires careful correction using standards or complex algorithms.
Surface-Level Analysis
XRF is fundamentally a surface-sensitive technique. The instrument's primary X-rays only penetrate a shallow depth into the sample, and the fluorescent X-rays can only escape from that same shallow layer. This means your analysis only represents the composition of the surface, which may not be representative of the bulk material if the sample is not perfectly homogenous.
Sample Homogeneity and Preparation
For the reasons above, sample quality is paramount. Inhomogeneous samples, rough surfaces, or variations in particle size can scatter X-rays unpredictably and lead to highly inaccurate results. For high-precision laboratory analysis, samples often must be ground into a fine powder and fused into a glass disc to create a perfectly flat and homogenous surface.
Understanding the Operational Trade-offs
Beyond the physics and the sample, practical considerations and operational requirements create another layer of challenges.
The Need for Expertise
Getting accurate data from an XRF instrument, especially for non-routine samples, requires a skilled operator. Recognizing potential spectral overlaps, designing proper calibration strategies, and understanding the impact of the sample matrix are not trivial tasks. As the references note, using XRF effectively requires the "right expertise."
Calibration Requirements
For accurate quantitative analysis, XRF instruments must be calibrated using standards that are very similar in composition to the unknown sample. Creating or acquiring these matrix-matched standards can be difficult and expensive, especially for unique or complex materials.
Instrumentation and Cost
There is a significant trade-off between performance and practicality. Handheld XRF (pXRF) analyzers offer incredible portability for field screening but have lower resolution and sensitivity. High-performance Wavelength Dispersive XRF (WDXRF) systems provide superior results but are large, expensive laboratory instruments that require a controlled environment.
Making the Right Choice for Your Goal
To use XRF effectively, you must align its capabilities with your analytical objective.
- If your primary focus is rapid screening of heavy metals: XRF is an excellent choice, but always be mindful that you are analyzing the surface and that matrix effects can influence quantification.
- If your primary focus is precise bulk composition of a known material: Laboratory XRF is a powerful tool, provided you invest in rigorous sample preparation to create a perfectly homogenous sample.
- If your primary focus is analyzing light elements (e.g., Lithium, Carbon, Oxygen): You must choose a different analytical technique, as XRF is not suitable for this task.
- If your primary focus is identifying chemical compounds or mineral phases: XRF is the wrong tool; techniques like X-Ray Diffraction (XRD) or Raman Spectroscopy are required.
Ultimately, XRF is a powerful technique when its limitations are respected and its application is appropriate for the problem at hand.
Summary Table:
| Problem Category | Key Issues | Impact on Analysis |
|---|---|---|
| Fundamental Limitations | Difficulty detecting light elements (below Sodium), spectral peak overlaps, limited chemical/oxidation state information. | Inaccurate or impossible analysis of light elements; potential misidentification/quantification of elements; no compound-specific data. |
| Sample-Related Challenges | Matrix effects (absorption/enhancement), surface-level analysis only, requires high homogeneity and specific preparation (e.g., grinding, fusion). | Quantification errors if not corrected; non-representative results for bulk material; increased time/cost for preparation. |
| Operational Trade-offs | Requires significant operator expertise, need for matrix-matched calibration standards, cost/performance balance (handheld vs. lab systems). | Risk of user error; expensive/ difficult calibration for complex samples; trade-off between portability and precision. |
Need precise elemental analysis for your lab? While XRF has limitations, KINTEK's expertise in lab equipment ensures you select the right tool for your specific needs—whether it's XRF for heavy metal screening or complementary techniques like XRD for compound identification. Our team provides tailored solutions, from high-performance WDXRF systems to sample preparation consumables, helping you achieve accurate, reliable results. Contact us today to discuss your application and optimize your analytical workflow! Reach out via our Contact Form
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Three-dimensional electromagnetic sieving instrument
- Automatic Laboratory Heat Press Machine
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
- Infrared Thermal Imaging Temperature Measurement Double-Sided Coated Germanium Ge Lens
People Also Ask
- How does an electrochemical workstation assist in assessing corrosion resistance? Quantify Laser-Remelted Steel Performance
- What is the significance of using a high-precision potentiostat for PDP scanning? Unlock Accurate Corrosion Insights
- How does a three-electrode electrochemical workstation assess TA10 titanium corrosion? Expert Testing Insights
- What is the operational mechanism of a three-electrode Electrochemical Workstation? Master Coating Corrosion Analysis
- What is the significance of Electrochemical Impedance Spectroscopy (EIS)? Decode Kinetics & Stability in Catalysts