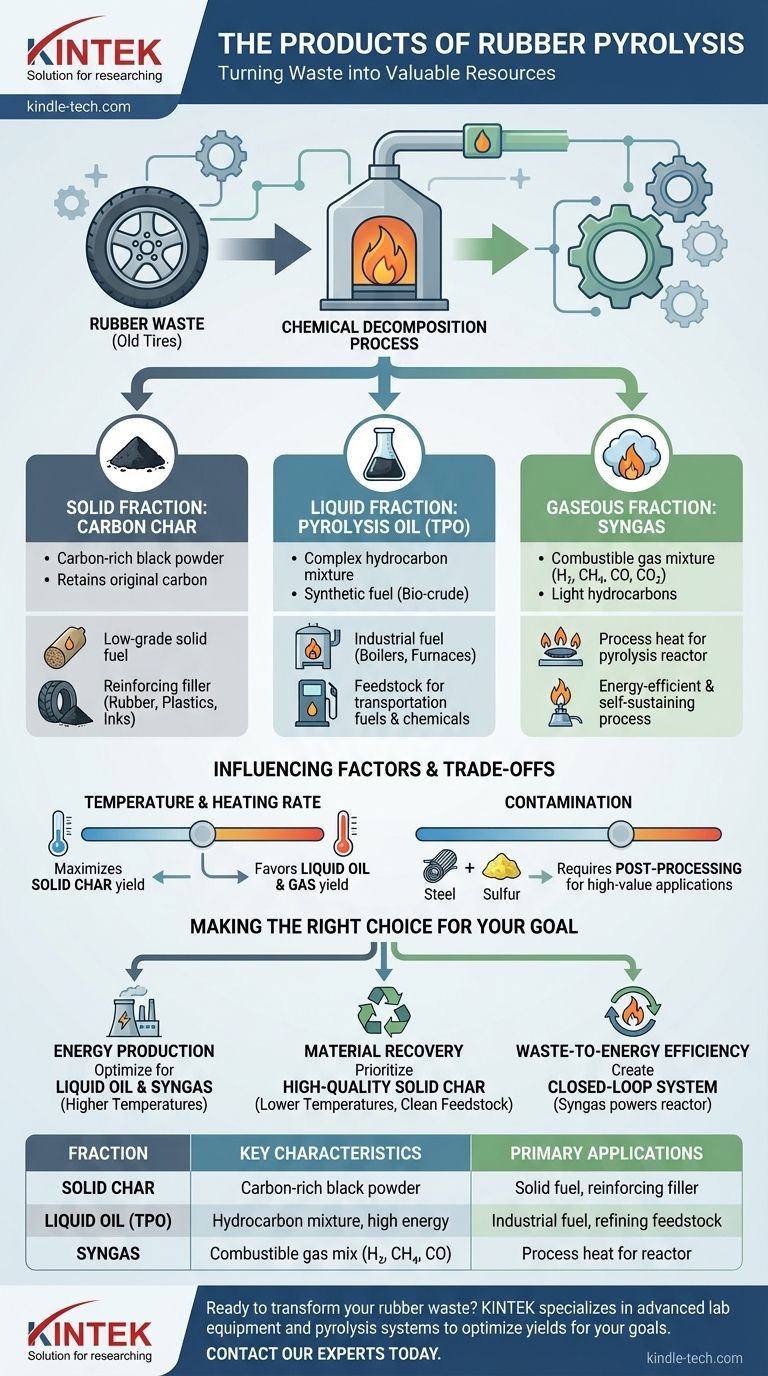
In short, the pyrolysis of rubber breaks it down into three distinct product categories: a solid char, a liquid oil, and a non-condensable gas. These outputs are not waste but rather recovered resources with significant potential value in industrial applications.
Pyrolysis is best understood not as a disposal method, but as a chemical decomposition process. It transforms a complex polymer waste stream (like old tires) into simpler, more valuable raw materials for energy and manufacturing.

Deconstructing the Pyrolysis Outputs
Pyrolysis subjects rubber to high temperatures in an oxygen-free environment. This thermal cracking breaks down the long polymer chains into the three primary fractions, each with its own characteristics and uses.
The Solid Fraction: Carbon Char
The solid residue left after pyrolysis is a form of carbon black, often referred to as char. It is a fine, black powder rich in carbon.
This material is not simply ash. It retains a significant portion of the original carbon from the rubber and can be used as a low-grade solid fuel or, after processing, as a reinforcing filler in new rubber products, inks, and plastics.
The Liquid Fraction: Pyrolysis Oil (TPO)
As the pyrolysis gas cools, a complex liquid mixture of hydrocarbons condenses out. This is known as Tire Pyrolysis Oil (TPO) or bio-crude.
This oil is a synthetic fuel with an energy value comparable to diesel. It can be used directly in boilers and furnaces or be further refined to produce transportation fuels and valuable chemical feedstocks.
The Gaseous Fraction: Syngas
The components that do not condense into liquid form a combustible gas mixture known as syngas (synthesis gas).
This gas is a blend of hydrogen (H₂), methane (CH₄), carbon monoxide (CO), carbon dioxide (CO₂), and various other light hydrocarbons. Because of its flammability, this gas is almost always captured and reused to provide the heat needed to power the pyrolysis reactor, making the entire process more energy-efficient and self-sustaining.
Understanding the Trade-offs and Influencing Factors
The exact ratio and composition of these three products are not fixed. They are highly dependent on the specific process conditions, which presents both a challenge and an opportunity.
The Impact of Temperature and Heating Rate
The final product yield can be intentionally manipulated. Lower temperatures (slow pyrolysis) tend to maximize the output of solid char, while higher temperatures and rapid heating rates (fast pyrolysis) favor the production of liquid oil and gas.
The Challenge of Contamination
The raw outputs from pyrolysis are not perfectly pure. The char may contain steel wire (from tires) and inorganic ash, while the oil can have a high sulfur content from the rubber vulcanization process. Both require post-processing and purification before they can be used in higher-value applications.
Process Viability
The economic and environmental viability of a pyrolysis plant depends on managing these variables effectively. Success requires optimizing the process for the most valuable local market—be it fuel oil, carbon black, or energy self-sufficiency—while implementing systems to clean and refine the resulting products.
Making the Right Choice for Your Goal
The optimal approach to rubber pyrolysis depends entirely on your end objective.
- If your primary focus is energy production: You should optimize for high yields of liquid pyrolysis oil and combustible syngas, which typically involves higher process temperatures.
- If your primary focus is material recovery: You should prioritize a high-quality solid char by using lower temperatures and ensuring the rubber feedstock is clean and well-prepared.
- If your primary focus is waste-to-energy efficiency: The main goal is to create a closed-loop system where the produced syngas provides all the heat required for the process.
Ultimately, rubber pyrolysis offers a powerful method for converting a problematic waste material into a valuable portfolio of resources.
Summary Table:
| Product Fraction | Key Characteristics | Primary Applications |
|---|---|---|
| Solid Char | Carbon-rich black powder | Solid fuel, reinforcing filler for rubber/plastics |
| Liquid Oil (TPO) | Hydrocarbon mixture, high energy value | Industrial fuel, feedstock for refining into diesel |
| Syngas | Combustible gas mix (H₂, CH₄, CO) | Process heat for the pyrolysis reactor itself |
Ready to transform your rubber waste into valuable resources? The pyrolysis process is a powerful solution, but its success depends on using the right equipment to optimize product yields for your specific goals—whether that's maximizing energy production or material recovery. KINTEK specializes in advanced lab equipment and pyrolysis systems to help you develop and refine your waste-to-resource strategy. Contact our experts today to discuss how our solutions can enhance your pyrolysis efficiency and product quality.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What is the effect of temperature on pyrolysis products? Master Product Yields with Thermal Control
- What is a rotary retort furnace? Achieve Superior Uniformity in Continuous Heat Treatment
- What are the different types of pyrolysis reactors? Choose the Right Reactor for Your Process
- What is the residence time of slow pyrolysis? Maximize Your Biochar Yield with Hours-Long Processing
- What is the main difference between gasification and pyrolysis? Choosing the Right Biomass Conversion Process
- What is the disposal of solid waste by pyrolysis? A Waste-to-Wealth Transformation Guide
- What is the industrial application of calcination? Transforming Raw Materials for Manufacturing
- What are the advantages of rotary kiln? Achieve Superior Uniformity in High-Temperature Processing



















